Figure 3.
KCC2 activation reduces neuronal Cl− accumulation and slows the development of seizure-like events in brain slices
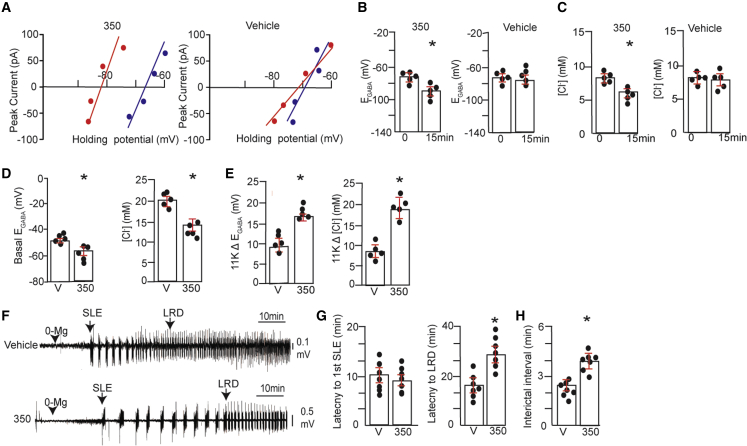
(A) 18–21 DIV hippocampal neurons were subjected to gramicidin perforated patch-clamp recordings in the presence of bumetanide (10 μM) and TTX (300 nM). After perforation, cultures were exposed to 300 nM 350 or V (1% BCD) for 15 min. EGABA was then determined using voltage ramps to determine the polarity of muscimol-induced currents, and representative I-V plots are shown for neurons at 0 (blue) and 15 (red) min treatment for V and 350.
(B) EGABA values were measured at 0 and 15 min following treatment with V or 300 nM 350 (n = 5 cultures).
(C) [Cl−] values were calculated from EGABA values for V- or 350-treated neurons (n = 5 cultures).
(D) 18–21 DIV hippocampal neurons were incubated with 300 nM 350 or V for 1 h. Neurons were subjected to whole-cell recording using an electrolyte containing 30 mM Cl−. 5 min later, basal EGABA, and [Cl−] were determined and compared between treatments (n = 5 cultures).
(E) Neurons treated as above were exposed to 11K (10 μM), and the shifts in EGABA (11KΔEGABA) and in [Cl−] (11KΔCl−) were compared (n = 5 cultures).
(F) Freshly prepared 350 μm brain slices from C57Bl/6 mice were incubated in ACSF supplemented with V or 350 (1 μM) for 1 h at 32°C and then with ACSF deficient in Mg+2 (0-Mg) (black arrowhead). Field recordings were then performed within the entorhinal cortex as a means of monitoring neuronal excitability. The first SLE and entrance into LRD development are indicated.
(G) The time to the first SLE and to LRDs were compared between treatments (n = 7 mice).
(H) The interictal interval was compared between the first and second SLE using slices treated with V or 350 (∗p < 0.05; n = 7 mice).
In all panels, p values were determined using t tests, ∗p < 0.05.