Abstract
Background.
The rising prevalence of insulin resistance (IR), metabolic syndrome and type 2 diabetes are associated with increases in abdominal mesenteric fat. Adipocytes are sensitive to low temperatures, making cryolipolysis of mesenteric fat an attractive treatment modality to potentially reduce IR.
Objectives.
We aimed to determine whether: 1. Cryolipolysis is safe in reducing the volume of the mesenteric fat; and 2. Reduction in mesenteric fat volume reduces indices of IR and glycemic dysfunction.
Setting.
Indiana University School of Medicine, USA.
Methods.
A novel cooling device and method delivered cryolipolysis in a controlled manner to avoid tissue ablative temperatures. Ossabaw pigs (n=8) were fed a high fat (HFD) diet for 9 months to develop visceral obesity, IR and metabolic syndrome. Following laparotomy, mesenteric fat cryolipolysis (MFC) was performed in 5 pigs, while 3 served as sham surgery control. The volume of the mesenteric fat was measured by computed tomography (CT) and compared to indices of glucose intolerance before, and at 3 and 6 months post-procedure.
Results.
MFC safely reduced mesenteric fat volume by ~30% at 3 months, which was maintained at 6 months. Body weight did not change in either the MFC or sham surgery control groups. Measure of glycemic control, insulin sensitivity and blood pressure significantly improved after MFC compared with sham controls.
Conclusions.
MFC reduces the volume of mesenteric fat and improves glycemic control in obese, IR Ossabaw pigs, without adverse effects.
Keywords: Mesenteric fat, Ossabaw swine, Insulin resistance, Cryolipolysis
Introduction.
The prevalence of type II diabetes mellitus (T2DM), insulin resistance, and obesity is continuously rising and poses a major risk for morbidity and mortality from cardiovascular and other complications (1,2). Studies in animal models and humans have shown a link between visceral (but not subcutaneous) fat and insulin resistance and related metabolic abnormalities (3–7). Among the fat depots, the mesentery stores most of the intra-abdominal fat, and has emerged as a key contributor to insulin resistance and diabetes via recruitment of inflammatory cells and the secretion of adipokines, cytokines, free fatty acids, and other diabetogenic factors (6–8). Moreover, increase in mesenteric fat thickness is closely linked to increased risk for developing the metabolic syndrome (MetS), defined as the presence of at least three of the following features: central obesity, high blood pressure, hyperglycemia, low high-density lipoprotein cholesterol (HDL-c), and elevated serum triglycerides (9,10). The direct drainage of the mesentery to the liver contributes to the development of fatty liver and liver-specific insulin resistance which in many cases antecede systemic insulin resistance and diabetes (5,7,8,11). Finally, the direct contribution of mesenteric fat to insulin resistance was recently demonstrated, where its reduction using a tissue liquidation technique on insulin resistant non-human primates served to improve insulin sensitivity and reduce body weight (6).
Mesenteric fat, per se, is unresectable surgically due to its vital neurovascular supply to the intestines, and targeting other abdominal visceral fat depots, such as the greater omentum, have proved to be suboptimal (12–16). The average amount of omentum resected in these studies was ~0.5–0.8 kg, which is lower than the average abdominal fat mass in obese individuals of >3 kg, with levels as high as ~7–8 kg in some individuals (17,18), and thus explains why these studies did not report a beneficial effect. The increased metabolic contribution of the mesenteric fat to diabetes, as opposed to omental or subcutaneous adipose tissue (7), further emphasizes the need to develop viable and effective methods for mesenteric fat reduction.
Adipocytes, due to their lipid content, are sensitive to low temperatures compared to other cell types, and induction of cell death can be detected at temperatures as high as +10°C (19). This relatively high temperature avoids tissue ablation or damage to other cells or adjacent vital structures. Thus, targeting fat cells for destruction using cold temperatures (cryolipolysis) is an attractive approach to induce mesenteric fat loss. Successful, practical delivery of a safe treatment that induces cryolipolysis of mesenteric fat (MFC) should demonstrate clinical efficacy by reducing insulin resistance and diabetes in patients with visceral adiposity.
Subcutaneous cryolipolysis using cooling plates placed externally on the skin is a method for fat reduction commonly used for cosmesis. It is durable and was shown in clinical studies to promote significant reduction in fat mass without significant side effects to surrounding tissue or changes in circulating lipid levels (19–22). Currently, there are no clinically available treatments to reduce the deleterious mesenteric fat mass, and alternate strategies which include dietary and lifestyle approaches are clearly not sustainable in most cases (23).
In this study, we sought to determine the practical feasibility, safety, and efficacy of reducing mesenteric fat volume using a novel cryolipolysis device as a new potential treatment to reduce insulin resistance and improve glucose regulation. We focused these studies in the Ossabaw pig model of the metabolic syndrome (MetS) induced by a diet high in calories, fat, fructose, and cholesterol (MetS diet). This model is widely used for studies of the MetS and represents a highly translatable model that develops each of the core parameters of the syndrome with many of the associated secondary comorbidities (24–28).
Methods.
Animal model.
All protocols involving animals were approved by the Indiana University Institutional Animal Care and Use Committee and complied fully with recommendations in the Guide for the Care and Use of Laboratory Animals (29) and the American Veterinary Medical Association Panel on Euthanasia (30).
MetS and insulin resistance were induced in 8 Ossabaw miniature swine (5 Males / 3 Females) with a daily atherogenic diet (1000g), high in fat, cholesterol and fructose for 9 months starting at 9 months of age. The diet provided 16.3% kcal from protein, 40.8% kcal from total carbohydrates (for which 19% kcal was fructose), and 42.9% kcal from fat. Fat calories were derived from a mixture of lard, hydrogenated soybean oil, and hydrogenated coconut oil. It was supplemented with 2.0% cholesterol and 0.7% sodium cholate by weight (KT324, Purina Test Diet, Richmond, IN) (31). All animals had free access to water.
Study design.
After 9 months of HFD, abdominal computed tomography (CT, described below) was performed to quantify the mesenteric fat. Blood was drawn and intravenous glucose tolerance test (IVGTT) was performed to establish baseline insulin resistance. The cryolipolysis of mesenteric fat procedure (MFC, described below) was performed on 5 pigs while 3 pigs underwent laparotomy and manipulation of their mesentery without cooling and served as sham controls. All surgical procedures and imaging as described below were identical between the groups, however, the cryolipolysis device was not activated in the sham group. Pigs were allowed to recover and monitored up to 6 months. CT and glucose monitoring were conducted at 3 and 6 months follow up. At 6 months, pigs were euthanized, and a necropsy was performed.
Cryolipolysis.
The pigs were randomly divided into sham (2M, 1F) and treated (3M/2F) groups. After an overnight fast, swine received a 2.2 mg/kg dose of xylazine (Webster Veterinary, Devens, MA) and 5.5 mg/kg dose of telazol (Fort Dodge Animal Health, Fort Dodge, IA) intramuscular injection to induce anesthesia. Swine were intubated and anesthesia was maintained with 2–4% isoflurane in 100% O2 as a carrier gas. The isoflurane level was adjusted to maintain anesthesia with stable hemodynamics. Heart rate, blood pressure, respiratory rate, and electrocardiographic data were continuously monitored throughout the procedure. A laparotomy was performed to expose the mesenteric fat of the small intestine. The average thickness of the mesenteric fat in these pigs was ~5mm, which is comparable to that of humans.
Cryolipolysis device.
A prototype cryolipolysis device was built by B2M Medical (Irvine, CA) for these early safety and efficacy studies. The device uses compressed nitrogen gas cooled to −190°C by liquid nitrogen and then heated to the treatment temperature by heating elements located in the device probe before being released through the probe nozzle. Based on preliminary bench testing and measurements of temperature penetration to fat tissue, we set the treatment parameters to cool the mesenteric fat to ≤10°C at a depth of 5mm. The target temperature was chosen based on studies that showed that adipocyte apoptosis can already be detected at 10°C (19,32). We identified the terminal ileum as the starting point for cryo-applications and treated the mesenteric fat in a retrograde direction throughout the small intestine until we reached the duodenum. Since the anatomy of the pig’s spiral colon does not allow access to its mesentery, we avoided treating the large intestine.
Computed tomography.
CT scans were conducted before cryolipolysis and at 3 and 6 months follow up to quantify changes in mesenteric fat, defined as fat engulfing the intestine excluding air (33,34). The pigs were scanned using a Philips Brilliance 64 detector scanner (Philips Healthcare, Andover, MA, USA and Best, NL) while sedated using 4% Isoflurane (Webster Veterinary, Devens, MA) supplied by mask. Helical scans were obtained craniocaudal from the sternal notch to the pubic symphysis, 20mm thick and taken every 0.5cm. Images were analyzed using Mimics Medical 24.0 (Materialize NV, Belgium), with Hounsfield units (HU) range of −150 to −20 (34). The mesenteric fat was segmented, and its volume (L) was calculated from the 3D representations.
Intravenous glucose tolerance test (IVGTT).
After an overnight fast, pigs were anesthetized with isoflurane and the right jugular vein was catheterized percutaneously (35). They were allowed to recover from anesthesia for at least 2 h before the IVGTT started, to avoid isoflurane induced decrease in insulin action (33). The animals were placed in a low-stress restraint sling and blood samples were obtained at baseline (−5 and 0 min), followed by an intravenous bolus of 1g glucose/kg body weight, and further samples were obtained at 5, 10, 20, 30, 40, 50, and 60 min after injection. Blood glucose was measured on a YSI 2300 Stat Plus analyzer (YSI, Yellow springs, OH). Plasma insulin levels were obtained by Elisa assays (Abcam, MA, USA) at 0, 10, 20, 40 and 60 min after injection of glucose. To assess insulin sensitivity, the products of plasma glucose and insulin levels were used to calculate a modified homeostatic model assessment of insulin resistance (HOMA-IR), and Quantitative Insulin Sensitivity Check Index (QUICKI) (33,36,37).
Blood pressure
Blood pressure during IVGTT was monitored using the cuff method as previously described (26), and values were computed from at least 6 different measurements over time.
Statistical Analysis:
Data presented as mean ± SEM unless otherwise indicated. The area under the curve (AUC) for glucose and insulin was calculated using the trapezoid method. 2-way anova for repeated measurements followed by post-hoc Bonferroni test for comparisons between groups was used to measure significance within groups. Student T test was used to compare values between groups at different time points. p<0.05 is considered as statistically significant.
Results.
Cryolipolysis.
All cryolipolysis and sham procedures were completed without any adverse events. Overall, we delivered ~40–60 applications per pig and the procedure time was ~90 minutes. No stigmata of intrabdominal bleeding were recorded, and all the pigs had regular bowel movements within 24h after the procedure. Three different designs of our prototype cryolipolysis probe allowed access to most of the visible mesenteric fat along the entire length of the small intestine (Figure 1A–C). Following cryo-application, the treated area underwent a transient pale discoloration with a firmer consistency compared to untreated tissue, until baseline body temperature was restored in that location (Figure 1D). We used this change in appearance to identify treated vs. untreated areas of the mesentery as we progressed to treat majority of the mesenteric surface.
Figure 1.
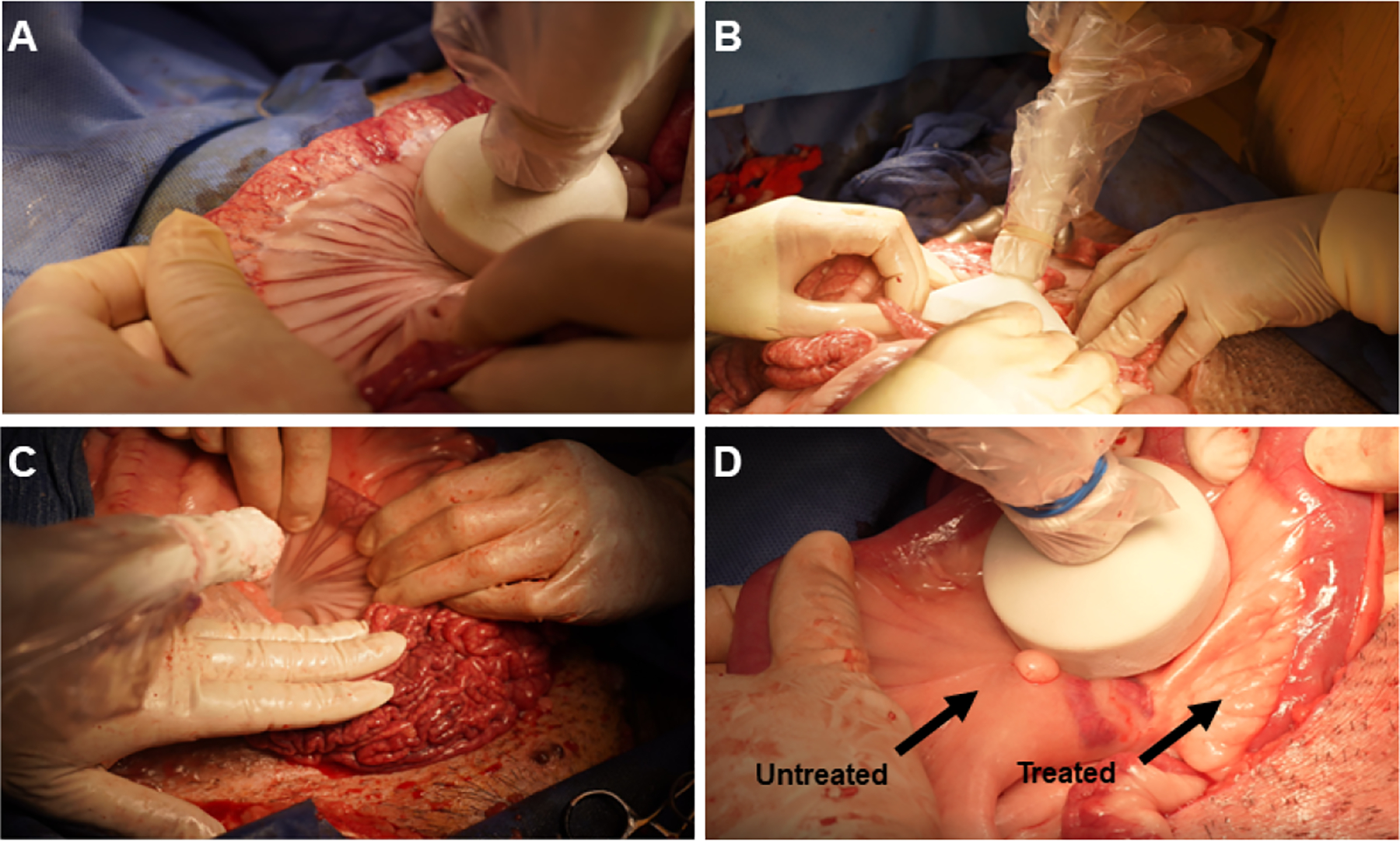
The cryolipolysis procedure in the Ossabaw pig. Mesenteric fat was exposed along the small intestine and treated with our prototype cryolipolysis device. A-C: We used three different probe designs to access most of the mesenteric fat (circular-big (A); rectangular (B) and circular-small (C)). D. The treated area became white and ridged following a treatment.
Mesenteric fat.
Before cryolipolysis (T=0), the mean volume of the mesenteric fat in the treated and sham groups was (2.98±0.23L vs 3.28± 0.87L, respectively; p=ns). At 3 months post-cryolipolysis, mesenteric fat volume was reduced by ~30% in the treated group, while it did not change significantly in the sham controls (2.22±0.19L vs. 3.26±0.9L, respectively; p=0.009). The reduction of mesenteric fat volume was sustained at 6 months (p=0.003) while the sham controls showed a 20% increase (2.03±0.22L vs 3.7±0.65L, respectively, Figure 2A+B). A representative segment of treated vs. untreated mesenteric fat taken at necropsy at 6 months shows a marked reduction in the thickness of the mesenteric fat following cryolipolysis (Figure 2C) without thrombotic or atrophic mesenteric vasculature.
Figure 2.
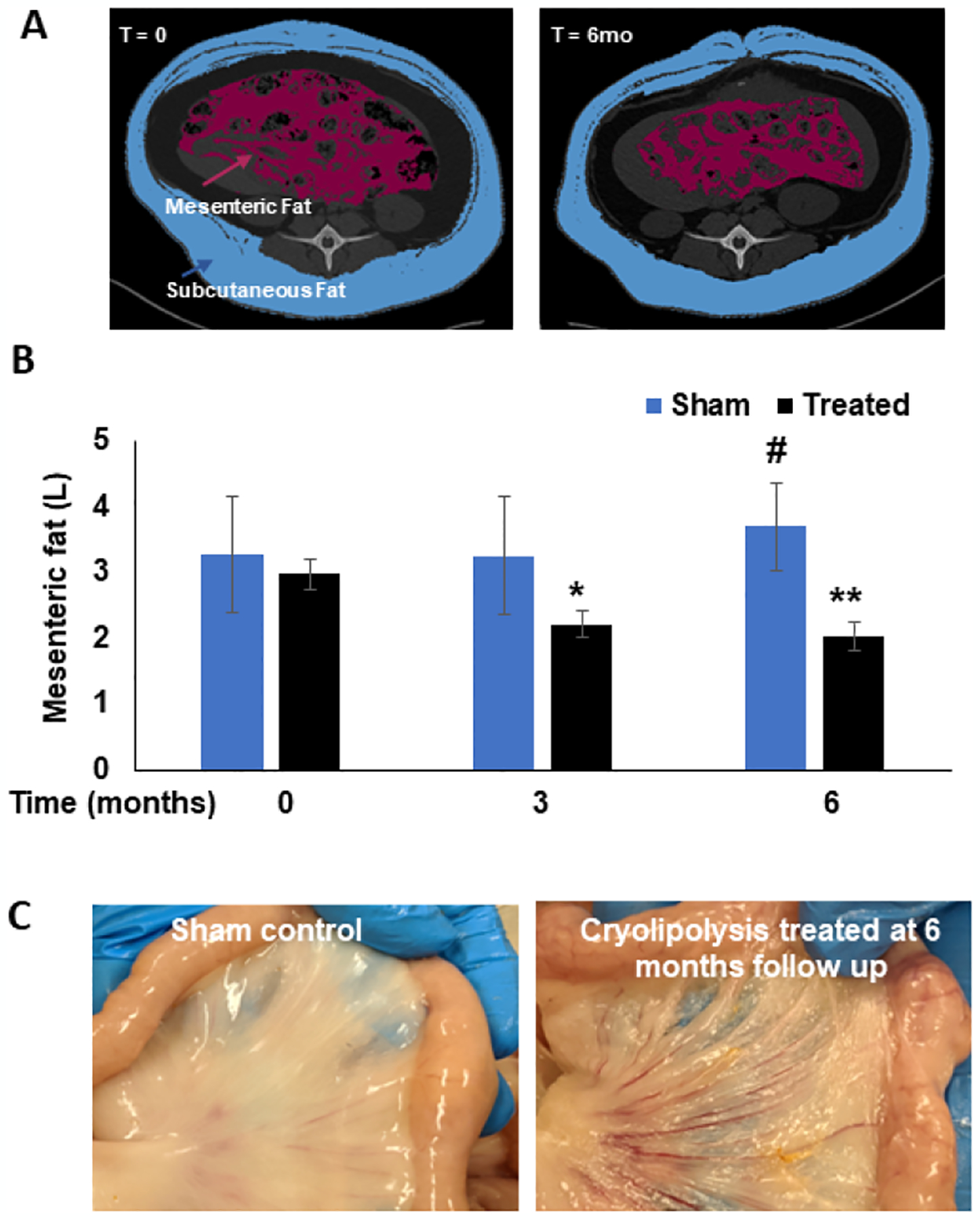
A. Representative CT images showing segmentation of the mesenteric fat (red) in a cryolipolysis treated pig before treatment (left) and the same pig at 6 months follow up (right). B. Volume of mesenteric fat in Sham (blue bars) and cryolipolysis-treated pigs (black bars) before treatment and at 3 and 6 months follow up. Data are presented as mean ± SEM. *P=0.009 treated at t=0 vs. t=3mo; **P=0.003 treated at t=0 vs. t=6mo. #P=0.01 sham vs. treated at t=6mo. C. Mesenteric fat of sham (left) and cryolipolysis-treated (right) at 6 months follow up, showing marked reduction in the thickness while no apparent damage to the microcirculation or the intestine.
Body weight.
Before the initiation of 9 months HFD, body weight was not different between the groups (32±4kg vs. 30±3kg, sham vs. treated, p=n.s). MFC had no significant effect on total body weight, which increased by ~4kg in both groups at 6 months follow-up (sham: 91.2±5.6 kg at t=0 to 95.1±3kg at t=6 months; treated: 84±6.5kg at t=0 to 87.1±3.5kg at t= 6 months; Figure 3A).
Figure 3.

(A) changes in total body weight and Systolic Blood Pressure (SBP) (B) in Sham and cryolipolysis treated before treatment (T=0) and at 3 and 6 months follow up. Data is expressed as mean ± SEM. For SBP: *P=0.02 treated at t=0 vs t=3mo; **P=0.007 treated at t=0 vs. t=6mo; #P=0.04 sham at t=0 vs t=6m; ## P= 0.0002 sham vs treated at t=6m. No significant changes in body weight between the groups in all time points.
Blood pressure.
Before the initiation of 9 months HFD, blood pressure was not different between the groups (109±5mmHg vs. 108±11 mmHg, sham vs. treated, p=n.s). Before cryolipolysis (T=0), both sham and treatment groups were hypertensive with systolic blood pressure (SBP) of 167±4mmHg and 183±22mmHg respectively. Mesenteric fat cryolipolysis reduced SBP in the treated group by 17% to 150±14mmHg at 3 months follow up (p=0.02), and these levels decreased further by 28% at 6 months follow up to 132±6mmHg (p=0.007). There was no significant change in SBP values in the sham control which remained elevated at 6 months follow up (183±23mmHg at 3 months and 187±7mmHg at 6 months; p=ns; Figure 3B).
Fasting Glucose and Insulin levels.
Before the initiation of 9 months HFD, fasting blood glucose was not different between the groups (64±7 mg/dL vs. 68±7 mg/dL, sham vs. treated, p=n.s). Before cryolipolysis (T=0), the mean values of fasting glucose and fasting insulin in the treated and sham groups were 89±11 and 99±4 mg/dL for glucose; 11.5±4.3 and 9±1.5 uU/ml for insulin, respectively; p=ns. At 3 months post cryolipolysis, these levels were reduced in the treated group to 75±8 mg/dL for glucose and 8.2±2.4 uU/ml for insulin (p<0.05). In the sham group, mean fasting glucose and insulin concentrations tended to increase at 3 and 6 months compared with values at baseline (T=0), but the differences were not statistically significant. In contrast, fasting plasma glucose and insulin concentrations were lower at 6 months compared with baseline in the MFC group (Figure 4A+B).
Figure 4. Cryolipolysis improves dysglycemia.

Fasting plasma glucose (A) and insulin (B) before treatment and at 3 and 6 months follow up. HOMA-IR (C) and QUICKI (D) scores were calculated as indicators of insulin resistance and sensitivity. Data are presented as mean ± SEM. For fasting glucose (A): *P=0.002 treated at t=0 vs. t=3mo; **P=0.04 treated at t=0 vs. t=6mo. #P=0.009 sham vs. treated at t=3mo; ##P=0.03 sham vs. treated at t=6mo. For fasting insulin (B): **P=0.02 treated at t=0 vs. t=6mo; #P=0.03 sham vs. treated at t=3mo; ##P=0.03 sham vs. treated at t=6mo. For HOMA-IR (C): *P=0.01 treated at t=0 vs. t=6mo; #P=0.04 sham vs. treated at t=3mo; ##P=0.04 sham vs. treated at t=6mo. For QUICKI (D): *P=0.01 treated at t=0 vs. t=6mo; #sham vs. treated at 6mo.
HOMA-IR and QUICKI scores.
Before cryolipolysis (T=0), the mean values of HOMA-IR and QUICKI were not significantly different between the sham and treated groups (HOMA-IR values: 1.98±0.32 and 1.72±0.2, p=ns; QUICKI(x1000): 346±9.36 and 339±14.53, p=ns, respectively). At 3 months post cryolipolysis, HOMA-IR values reduced to 1.61±0.57 in the treated group while increased to 3.83±1.51 in the sham control (p<0.05). At 6 months, HOMA-IR further reduced in the treated group to 1.0±0.34 (p<0.05), while it remained elevated at 3.5± 1.51 in the sham control. QUICKI score increased at 3 months in the cryolipolysis treated group while it remained reduced in the sham control [368±15.65 vs. 325±23.23 respectively]. At 6 months follow up, QUICKI further increased in the treated group to 406±27.42 (p=0.01) while it was unchanged in the sham control (330±22.88; Figure 4 C+D).
Glucose and Insulin levels during IVGTT.
Glucose: Before cryolipolysis (T=0), the peak value as well as the area under the curve (AUC) for glucose were not significantly different between the sham and treated groups (AUC values: 32402±908.23 vs. 29169±1162 respectively; p=ns). At 3 months post mesenteric fat cryolipolysis, both the peak values and the AUC for glucose were reduced compared to the sham control (AUC values: 26527±2054 vs. 35764±1382, p=0.009). These reduced values were also sustained at 6 months follow up (AUC values: 25452±1890 vs 33275±1869; treated vs. sham, respectively; p=0.01) (Figure 5A+B). Insulin: Before cryolipolysis (T=0), the peak value as well as the area under the curve (AUC) for insulin were not significantly different between the sham and treated groups (AUC values: 4218±787 vs. 3772±521 respectively; p=ns). Three months following mesenteric fat cryolipolysis, both the peak values and the AUC for insulin were reduced compared to the sham control (AUC values: 2096±101 vs. 3598±989). These reduced values were sustained at 6 months follow up (AUC values:1989±200 vs. 3456±741; p=0.02, Figure 5C+D).
Figure 5.
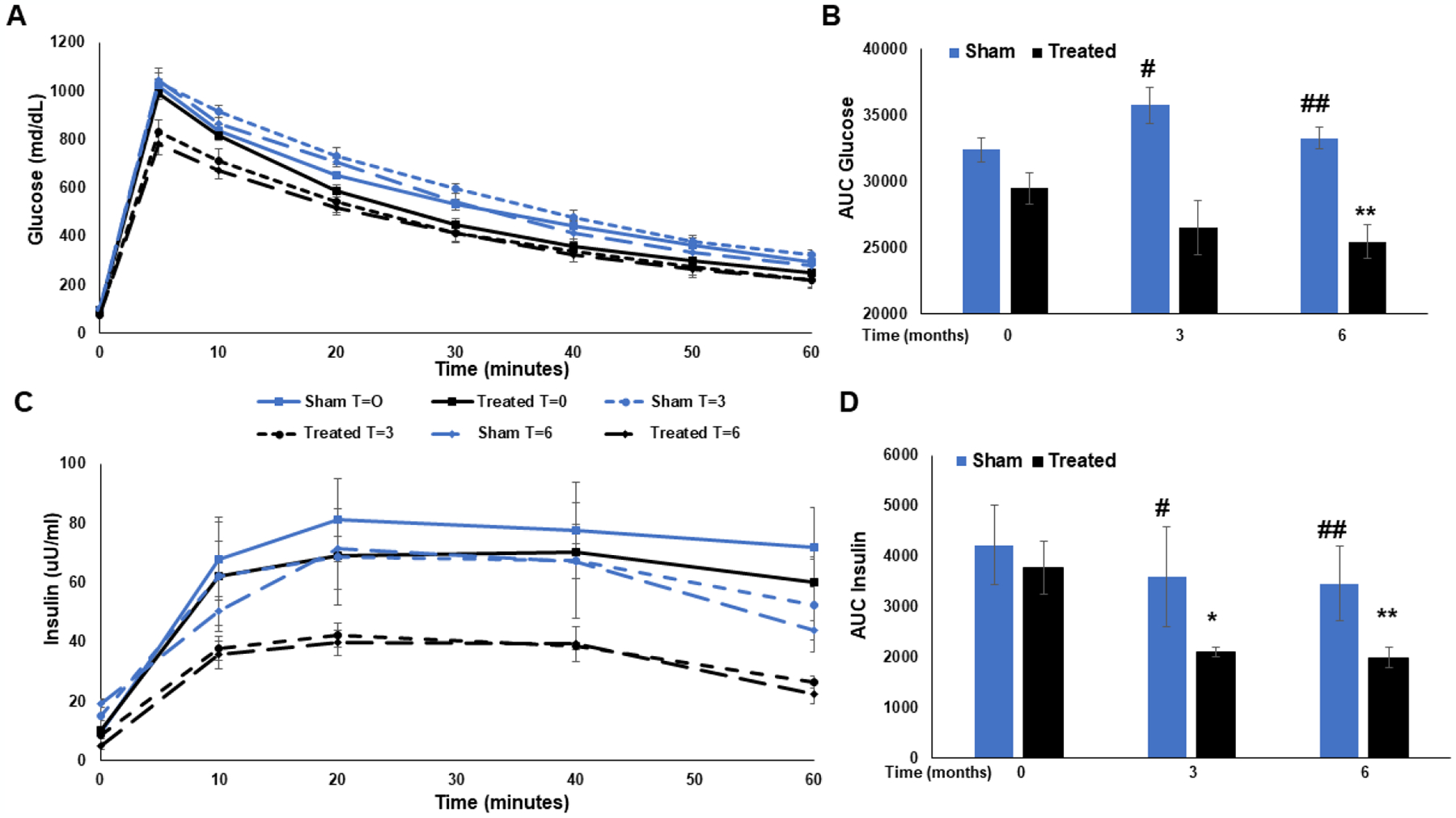
Dynamic glucose and insulin levels during 1-hour IVGTT. A. Plasma glucose levels were measured before glucose administration and at 5, 10, 20, 30, 40, 50 and 60 min thereafter for both groups before cryolipolysis and at 3 and 6 months follow up. The area under the curve was calculated for each group at the respected follow up period and is presented in B. C. Plasma insulin levels were measured before glucose administration and at 10, 20, 40, and 60 min thereafter for both groups before cryolipolysis and at 3 and 6 months follow up. The area under the curve was calculated for each group at the respected follow up period and is presented in D. Data is presented as mean ± SEM. For Glucose AUC: **P=0.01 treated at t=0 vs. t=6mo; #P=0.009 sham vs. treated at 3mo; ##P=0.01 sham vs. treated at 6mo. for insulin AUC: *P=0.03 treated at t=0 vs. t=3mo; **P=0.02 treated at t=0 vs. t=6mo. #P=0.03 sham vs. treated at 3mo. ##P=0.02 sham vs. treated at 6mo.
Safety and Pathology.
There were no adverse events at all stages of the study in response to MFC. All pigs recovered from the procedure with no difference in food/water consumption or bowel movements compared to the sham controls. At necropsy, a subjective, relative score assessing the degree of fibrous adhesions in the mesentery was performed by a pathologist. The score ranged from 0 (no adhesions) to 10 (widespread) and all the pigs were scored between 0–3. Gross pathology and histology showed minor local adhesions (in both groups) along the intestine with no apparent physiological relevance (Figure 6). Examination of internal organs such as adjacent bowel, heart, liver, kidney, and spleen showed no signs of damage, and they were comparable to sham controls.
Figure 6.
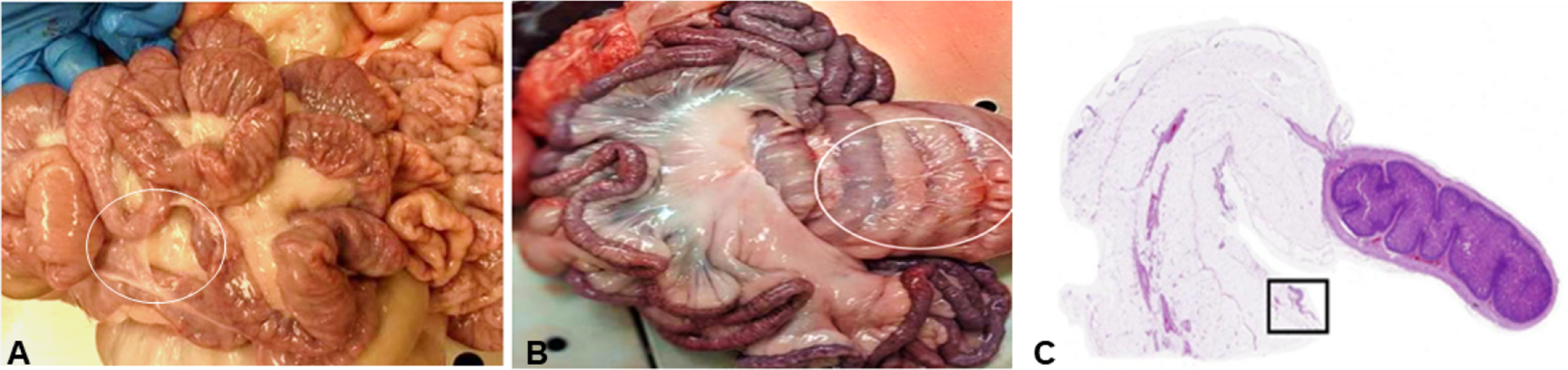
Gross photographs of the intestine taken at 6 months follow up, showing minor local adhesions on the intestine serosa (white circle) in both cryolipolysis treated (A) and (B) sham control. (C) Sub-gross histology. H&E of mesenteric fat and the small intestine cross section from a cryolipolysis treated pig at 6 months follow up. The mesentery features minimal areas of benign fibrous adhesions on the surface (boxed area). Bar=5mm.
Discussion.
In the present study, we demonstrated the safety and therapeutic metabolic efficacy of mesenteric fat cryolipolysis on glycemic control and indices of insulin resistance in the Ossabaw swine model of HFD induced MetS. We found MFC can decrease the volume of the mesenteric fat by ~30% at 3 months follow up, which was sustained at 6 months post procedure and was associated with a decrease in BP, fasting blood glucose, glucose AUC after glucose infusion and insulin resistance. Moreover, the cryolipolysis procedures produced no early or late complications with all animals surviving to the termination of the study. To our knowledge, this is the first report of the safe reduction of mesenteric fat using cold temperatures as an efficient treatment for insulin resistance and MetS.
Excess visceral adipose tissue is associated with an increased risk for metabolic disorders, including diabetes and MetS and increased morbidity and mortality from cardiovascular complications (38,39). Studies of visceral fat in both human and animal models have shown improvement in MetS and diabetes with dietary reduction of this depot (40–42). Notably however, the surgical resection of visceral fat in human omentum has shown mixed results with regards to reversing insulin resistance (14–16).This could be explained by the fact that the mesentery, which stores most of the abdominal fat, could not be surgically removed without compromising the vitality of the intestines.
In obese patients with diabetes, the obesity-related gene expressions in the mesenteric adipose tissue are up regulated, suggesting that mesenteric adipose depot may play a critical role in insulin resistance of type 2 diabetes. Moreover, it was suggested that increased lipid accumulation in the mesentery contributes to fatty liver and insulin resistance (7,8). Recently, a role in promoting insulin resistance was demonstrated in baboons, where partial removal of mesenteric fat using a tissue liquidation technique reversed insulin resistance and promoted weight loss (6). Overall, these studies highlight mesenteric fat as a metabolically active organ which, when dysfunctional, may promote the metabolic syndrome and progression to type 2 diabetes. Hence, harnessing therapies targeting this organ may add more tools for confronting metabolic disease resulting from mesenteric adiposity and dysglycemia.
Subcutaneous cryolipolysis is a procedure that removes subcutaneous fat, is widely used for cosmesis, and has been shown to be durable and safe (19,22). The approach utilizes the sensitivity of fat cells to low temperatures and the fact that apoptosis occurs at temperatures of 10°C and lower (18,25,26). While other cell types tolerate decreased temperatures, fat cells, when exposed to cold, undergo solidification of their lipid content and form needle-like crystals that puncture the cell membrane, thereby promoting cell death. Inflammation in the treated sites increase local cytokine production and provide additional fat cell loss overtime, after hypothermic exposure (18,26).
Following cryolipolysis, we found on average a ~30% decrease in mesenteric fat volume measured at 3- and 6-months post procedure. This amount of fat loss was sufficient to demonstrate a durable reduction in glucose dysregulation indices, namely fasting glucose, and insulin levels, as well as glucose and insulin concentrations during the intravenous glucose tolerance test, without a decrease in body weight.
An interesting and somewhat unexpected result was the reduction in blood pressure following the procedure. This reduction was not associated with any appreciable reduction in total body weight and, to our knowledge, this effect of mesenteric fat reduction on hypertension has not been reported. The association between visceral obesity and hypertension, both components of the MetS, is well established (43). Studies have shown that increased adipose tissue contributes to increased vascular resistance and dysfunction of the mesenteric lymphatic system, all of which contribute to chronic inflammation and glucose dysregulation (44,45). Thus, it is plausible that by cooling and reducing mesenteric fat, we also initiated a reduction in inflammatory mediators that promote vasoconstriction and hypertension. Other possible contributing mediators of hypertension in obesity include the direct effects of elevated leptin and insulin (46,47). Although we showed a reduction of insulin after mesenteric cryolipolysis, we did not measure leptin levels. Leptin activates the sympathetic nervous system and if cryolipolysis reduces leptin levels, reduced sympathetic tone may be another potential mechanism for the observed reductions of blood pressure.
Finally, we found that mesenteric fat cryolipolysis did not result in weight loss, as all the animals modestly gained weight by the 6 months follow up. These results differ from the findings in a study that extracted mesenteric fat in insulin resistant baboons, that found reduced insulin resistance concomitantly with reduction in food intake and body weight (6). One possible explanation may be the differences between species. While the baboons decreased food intake following surgery, our pigs consumed all their daily feed within 24h of their procedures. Thus, our results show that improvements in glycemic control after mesenteric fat cryolipolysis are achieved independently from weight reduction when applied to obese pigs. We do however expect that in human subjects with visceral obesity, some weight reduction will occur in alignment with the improvement in the overall metabolic status of the patient.
Our study was a “proof of concept”, showing that MFC is a safe and effective method to remove mesenteric fat, and that this reduction will promote sustained improvements in glycemic control. It remains to be determined how much mesenteric fat reduction is desired in order to reach clinically significant reductions in insulin resistance and features of the MetS in humans. To answer that question, dose-efficacy studies are proposed to create a personalized treatment protocol. It is possible that mesenteric fat cell destruction could release fatty acids into the portal vein and cause an accumulation of intrahepatic fat. While we measured plasma lipids at serial timepoints and found no increase in levels of triglycerides, HDLc or LDLc, a dedicated analysis of hepatic fat content following mesenteric fat cryolipolysis will be needed in the future. Finally, we found mesenteric fat reduction to be durable at 6 months follow up, however, both treated and sham groups gained weight. Since the diet did not change during the follow up period, this result could be explained by fat redistribution to other fat depots such as subcutaneous fat. Thus, we used our CT scans to measure the volume of the subcutaneous fat around the abdomen area and found no differences between the time points. Other parts which are known in pigs to accumulate subcutaneous fat like the neck and limbs were not scanned. Thus, it is plausible to hypothesis that in pigs, reduction in mesenteric fat promoted fat redistribution to other parts, mainly subcutaneous depots, however, fat redistribution following mesenteric fat cryolipolysis merits further investigation.
Perspectives.
We showed in the Ossabaw swine, which represents a highly translatable model that develops each of the core parameters of the MetS with many of the associated secondary comorbidities (27,28), that delivering non-ablative cold temperatures to the mesenteric surface promotes fat loss concomitant with reduction in insulin resistance and MetS indices. Moreover, we showed that in pigs, the procedure is safe and durable for at least 6 months. With the increased global incidence of insulin resistance and diabetes, together with poor medication adherence reported for many patients, new treatment modalities are needed to reverse insulin resistance and reduce the risk for future complications (48–50). While we utilized the laparotomy approach in these early feasibility studies to access the mesenteric fat, MFC is envisioned to rapidly transition to a laparoscopic approach and delivery, further reducing pain and potential risks. The optimal minimal dose-effect, efficacy, safety, and durability of the proposed treatment in humans, remain to be determined.
Highlights:
Excess mesenteric fat contributes to insulin resistance and metabolic syndrome
Cryolipolysis is effective in reducing the volume of mesenteric fat
In a pig model, mesenteric fat cryolipolysis improved glycemic control and reduced blood pressure
The procedure was well tolerated, and no safety issues were recorded
Acknowledgement:
“Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number R43DK133015 (to Mazor R).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: All co-authors have filled the ICMJE COI form.
References
- 1.Bhupathiraju SN, Hu FB. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. circ res. 2016;27(118):1723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. 2012. Apr;19(2):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, et al. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999. Jan;48(1):94–8. [DOI] [PubMed] [Google Scholar]
- 4.Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002. Oct;51(10):2951–8. [DOI] [PubMed] [Google Scholar]
- 5.Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, van Citters GW, Dea MK, et al. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2005;288(2):e454–61. [DOI] [PubMed] [Google Scholar]
- 6.Andrew MS, Huffman DM, Rodriguez-Ayala E, Williams NN, Peterson RM, Bastarrachea RA. Mesenteric visceral lipectomy using tissue liquefaction technology reverses insulin resistance and causes weight loss in baboons. Surgery for Obesity and Related Diseases. 2018. Jun;14(6):833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YK, Chen M, Clements RH, Abrams GA, Aprahamian CJ, Harmon CM. Human mesenteric adipose tissue plays unique role versus subcutaneous and omental fat in obesity related diabetes. Cell Physiol Biochem. 2008;22(5–6):531–8. [DOI] [PubMed] [Google Scholar]
- 8.Wueest S, Item F, Lucchini FC, Challa TD, Müller W, Blüher M, et al. Mesenteric Fat Lipolysis Mediates Obesity-Associated Hepatic Steatosis and Insulin Resistance. Diabetes. 2016. Jan;65(1):140–8. [DOI] [PubMed] [Google Scholar]
- 9.Hung Liu K, Leung Chan Y, Bun Chan W, Chung Ngor Chan J, Wing Winnie Chu C. Mesenteric Fat Thickness Is an Independent Determinant of Metabolic Syndrome and Identifies Subjects With Increased Carotid Intima-Media Thickness. Vol. 29, DIABETES CARE. 2006. [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel on Detection E and T of HBC in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001. May 16;285(19):2486–97. [DOI] [PubMed] [Google Scholar]
- 11.Klein S The case of visceral fat: argument for the defense. J Clin Invest. 2004. Jun;113(11):1530–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thörne A, Lönnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002. Feb;26(2):193–9. [DOI] [PubMed] [Google Scholar]
- 13.Csendes A, Maluenda F, Burgos AM. A prospective randomized study comparing patients with morbid obesity submitted to laparotomic gastric bypass with or without omentectomy. Obes Surg. 2009. Apr;19(4):490–4. [DOI] [PubMed] [Google Scholar]
- 14.Dillard TH, Purnell JQ, Smith MD, Raum W, Hong D, Laut J, et al. Omentectomy added to Roux-en-Y gastric bypass surgery: a randomized, controlled trial. Surg Obes Relat Dis. 9(2):269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010. Aug;139(2):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera MF, Pantoja JP, Velázquez-Fernández D, Cabiedes J, Aguilar-Salinas C, García-García E, et al. Potential additional effect of omentectomy on metabolic syndrome, acute-phase reactants, and inflammatory mediators in grade III obese patients undergoing laparoscopic Roux-en-Y gastric bypass: a randomized trial. Diabetes Care. 2010. Jul;33(7):1413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neeland IJ, Grundy SM, Li X, Adams-Huet B, Vega GL. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas Heart Study. Nutr Diabetes. 2016. Jul;6(7):e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salman AA, Salman MA, Said M, el Sherbiny M, Elkassar H, Hassan MB, et al. Improvement of Pancreatic Steatosis and Indices of Insulin Resistance After Metabolic Surgery. Front Med (Lausanne). 2022;9:894465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manstein D, Laubach H, Watanabe K, Farinelli W, Zurakowski D, Anderson RR. Selective cryolysis: A novel method of non-invasive fat removal. Lasers in Surgery and Medicine. 2008. Nov;40(9):595–604. [DOI] [PubMed] [Google Scholar]
- 20.Coleman SR, Sachdeva K, Egbert BM, Preciado J, Allison J. Clinical efficacy of noninvasive cryolipolysis and its effects on peripheral nerves. Aesthetic Plast Surg. 2009. Jul;33(4):482–8. [DOI] [PubMed] [Google Scholar]
- 21.Derrick CD, Shridharani SM, Broyles JM. The Safety and Efficacy of Cryolipolysis: A Systematic Review of Available Literature. Aesthet Surg J. 2015. Sep;35(7):830–6. [DOI] [PubMed] [Google Scholar]
- 22.Ingargiola MJ, Motakef S, Chung MT, Vasconez HC, Sasaki GH. Cryolipolysis for Fat Reduction and Body Contouring: Safety and Efficacy of Current Treatment Paradigms. Plastic and Reconstructive Surgery. 2015. Jun 25;135(6):1581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall KD, Kahan S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med Clin North Am. 2018. Jan;102(1):183–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturek M, Tune JD AM. Ossabaw Island miniature swine: metabolic syndrome and cardiovascular assessment. In: Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. 2015. p. 451–65. [Google Scholar]
- 25.Sham JG, Simianu V v, Wright AS, Stewart SD, Alloosh M, Sturek M, et al. Evaluating the mechanisms of improved glucose homeostasis after bariatric surgery in Ossabaw miniature swine. J Diabetes Res. 2014;2014:526972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neeb ZP, Edwards JM, Alloosh M, Long X, Mokelke EA, Sturek M. Metabolic syndrome and coronary artery disease in Ossabaw compared with Yucatan swine. Comp Med. 2010. Aug;60(4):300–15. [PMC free article] [PubMed] [Google Scholar]
- 27.Cluzel GL, Ryan PM, Herisson FM, Caplice NM. High-fidelity porcine models of metabolic syndrome: a contemporary synthesis. Am J Physiol Endocrinol Metab. 2022;322(4):E366–81. [DOI] [PubMed] [Google Scholar]
- 28.Sturek M, Alloosh M, Sellke FW. Swine Disease Models for Optimal Vascular Engineering. Annu Rev Biomed Eng. 2020;22:25–49. [DOI] [PubMed] [Google Scholar]
- 29.Washington DC: NAP. Institute for Laboratory Animal Research: Guide for the care and use of laboratory animals. 8th ed. 2010. [Google Scholar]
- 30.AVMA Panel on Euthanasia: American veterinary medical association: 2000 report of the AVMA panel on euthanasia. JAVMA. 2001;218:669–96. [DOI] [PubMed] [Google Scholar]
- 31.McKenney ML, Schultz KA, Boyd JH, Byrd JP, Alloosh M, Teague SD, et al. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg. 2014. Jan;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki GH, Abelev N, Tevez-Ortiz A. Noninvasive selective cryolipolysis and reperfusion recovery for localized natural fat reduction and contouring. Aesthet Surg J. 2014. Mar;34(3):420–31. [DOI] [PubMed] [Google Scholar]
- 33.Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of Metabolic Syndrome and Coronary Artery Disease in Female Ossabaw Swine Fed Excess Atherogenic Diet. Comparative Medicine. 2006;56(1):35–45. [PubMed] [Google Scholar]
- 34.Chang J, Jung J, Lee H, Chang D, Yoon J, Choi M. Computed tomographic evaluation of abdominal fat in minipigs. Journal of Veterinary Science. 2011;12(1):91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carroll JA, Daniel JA, Keisler DH, Matteri RL. Non-surgical catheterization of the jugular vein in young pigs. Lab Anim. 1999;33(2):129–34. [DOI] [PubMed] [Google Scholar]
- 36.Stern SE WK, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005;54(2):333–9. [DOI] [PubMed] [Google Scholar]
- 37.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000. Jul;85(7):2402–10. [DOI] [PubMed] [Google Scholar]
- 38.Klein S, Gastaldelli A, Yki-Järvinen H, Scherer PE. Why does obesity cause diabetes? Vol. 34, Cell Metabolism. Cell Press; 2022. p. 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fruh SM. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017;29(1):s3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006. Dec 14;444(7121):881–7. [DOI] [PubMed] [Google Scholar]
- 41.Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care. 2005. Sep;28(9):2322–5. [DOI] [PubMed] [Google Scholar]
- 42.Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–25. [DOI] [PubMed] [Google Scholar]
- 43.Sironi AM, Gastaldelli A, Mari A, Ciociaro D, Positano V, Postano V, et al. Visceral fat in hypertension: influence on insulin resistance and beta-cell function. Hypertension. 2004. Aug;44(2):127–33. [DOI] [PubMed] [Google Scholar]
- 44.Cao E, Watt MJ, Nowell CJ, Quach T, Simpson JS, de Melo Ferreira V, et al. Mesenteric lymphatic dysfunction promotes insulin resistance and represents a potential treatment target in obesity. Nature Metabolism. 2021. Sep 1;3(9):1175–88. [DOI] [PubMed] [Google Scholar]
- 45.Gálvez B, de Castro J, Herold D, Dubrovska G, Arribas S, González MC, et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006. Jun;26(6):1297–302. [DOI] [PubMed] [Google Scholar]
- 46.Poetsch MS, Strano A, Guan K. Role of Leptin in Cardiovascular Diseases. Front Endocrinol (Lausanne). 2020;11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zamami Y, Takatori S, Hobara N, Yabumae N, Tangsucharit P, Jin X, et al. Hyperinsulinemia induces hypertension associated with neurogenic vascular dysfunction resulting from abnormal perivascular innervations in rat mesenteric resistance arteries. Hypertens Res. 2011. Nov;34(11):1190–6. [DOI] [PubMed] [Google Scholar]
- 48.Aloudah NM, Scott NW, Aljadhey HS, Araujo-Soares V, Alrubeaan KA, Watson MC. Medication adherence among patients with type 2 diabetes: A mixed methods study. PLoS ONE. 2018. Dec 1;13(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee CS, Tan JHM, Sankari U, Koh YLE, Tan NC. Assessing oral medication adherence among patients with type 2 diabetes mellitus treated with polytherapy in a developed Asian community: A cross-sectional study. BMJ Open. 2017. Sep 1;7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim YY, Lee JS, Kang HJ, Park SM. Effect of medication adherence on long-term all-causemortality and hospitalization for cardiovascular disease in 65,067 newly diagnosed type 2 diabetes patients. Scientific Reports. 2018. Dec 1;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]


