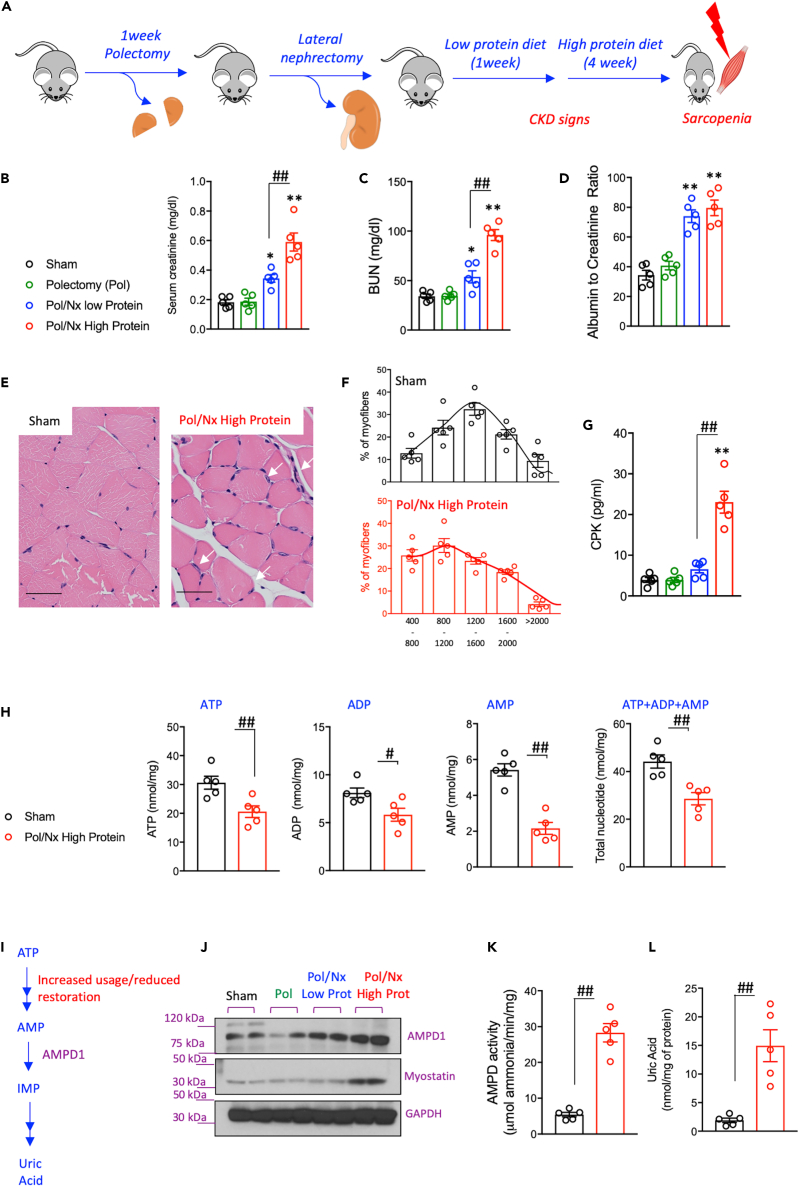
Figure 1.
CKD-dependent muscle atrophy is associated with nucleotide turnover and AMPD1 activation
(A) Schematic of the mouse model employed to induce CKD-dependent muscle atrophy.
(B–D) Blood creatinine, urea and urinary albumin excretion in wild type mice undergoing sham operation (black) or at different states of the model: polectomy (green), lateral nephrectomy and low protein diet (green) and lateral nephrectomy and high protein diet (red).
(E–G) (E) Representative H&E image of gastrocnemius of mice undergoing sham or with CKD. White arrows denote inflammatory cells. Bar = 25 μm (F) Cross-sectional analysis of myofiber sizes in sham and CKD mice showing a shift to the left (smaller size) (150 myofibers/muscle measured) (G) Plasma CPK levels in mice from same groups as in B).
(H) Intramuscular nucleotide pool (ATP, ADP, AMP and total nucleotides) in sham and CKD mice.
(I) Schematic of AMP metabolism via AMPD1 after ATP metabolism.
(J) Representative western blot for AMPD1 and myostatin in gastrocnemius of mice from the same groups as in B).
(K and L) Intramuscular AMPD activity and uric acid levels in sham and CKD mice. Statistical analysis: B-D and G) One way ANOVA followed by Tukey’s multiple comparison tests. H,K-L) two-tail t-test. ∗p <0.05 and ∗∗p <0.01 versus sham. #p <0.05 and ##p <0.01 n = 5 mice per group.