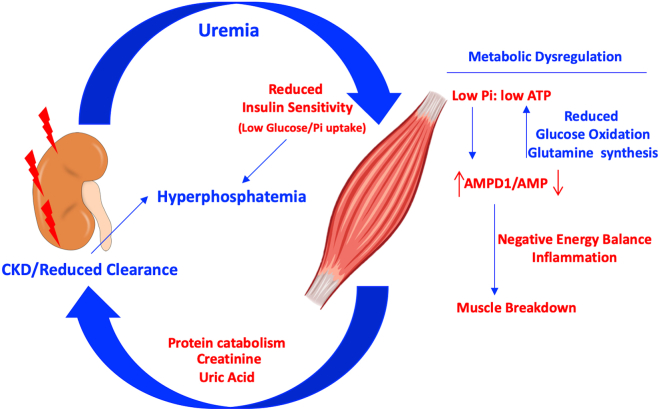
Figure 5.
Potential mechanism or kidney-muscle interplay in the progression of CKD and sarcopenia
In CKD, reduced clearance elevates plasma levels of urea. In turn, increased uremia impairs insulin action on the skeletal muscle leading to a reduction of both insulin-dependent glucose and phosphate uptake causing metabolic dysregulation. Hyperphosphatemia is then the consequence of both reduced renal clearance and low uptake by the skeletal muscle. Metabolic dysregulation in the skeletal muscle is characterized by low phosphate, reduced glucose oxidation and protein synthesis and overall low ATP and phosphocreatine levels with AMPD1 activation. Hyperactive AMPD1, in turn, decreases the amount of AMP while promoting the formation of uric acid in the purine degradation pathway. As a result of a negative energy balance in the muscle, inflammation and protein turnover is manifest thus leading to muscle atrophy. In consequence, muscle-derived products from protein catabolism and AMPD1 activation are released to the circulation to further contribute to the progression of CKD.