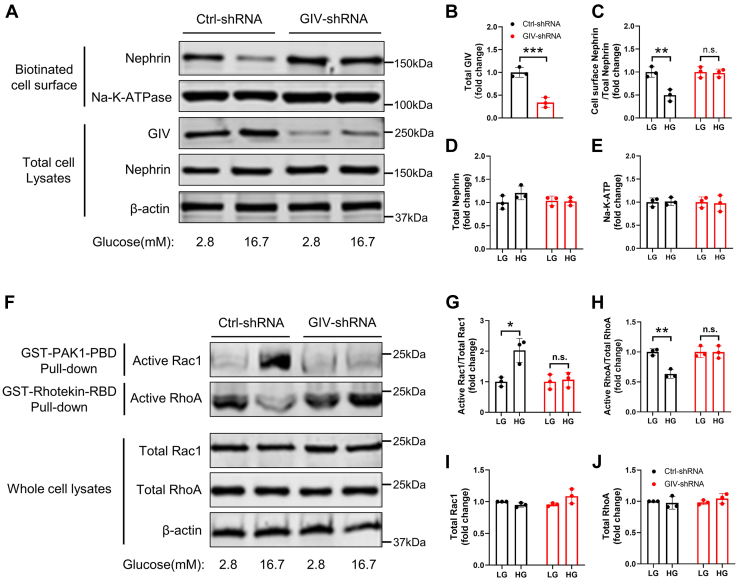
Figure 5.
GIV modulated F-actin remodeling through mediating endocytosis of Nephrin after high-glucose stimulation.A, subcellular expression of Nephrin on the plasma membrane in glucose-induced GIV downregulated MIN6 cells was checked by cell-surface biotinylation assay. B–E, the protein levels normalized by those of β-actin were measured by densitometry (n = 3). F, MIN6 cells were infected with shRNA for 48 h, and 2.8 mM glucose and 16.7 mM glucose were added for 30 min before cells were lysed. Cell lysates were subjected to GST-PAK1-PBD (GTP bound [active form] Rac1 interaction binding) or GST-Rhotekin-RBD (GTP bound [active form] RhoA interaction binding) pull-down followed by immunoblotting with anti-Rac1 and anti-RhoA. Normalized to total Rac1 and RhoA. G–J, the expression levels of active Rac1 and active RhoA normalized by their total protein levels were measured by densitometry, and the total protein levels of Rac1 and RhoA were normalized by those of β-actin (n = 3). The statistical significance of differences between means was assessed by the Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; n.s. means not significant.