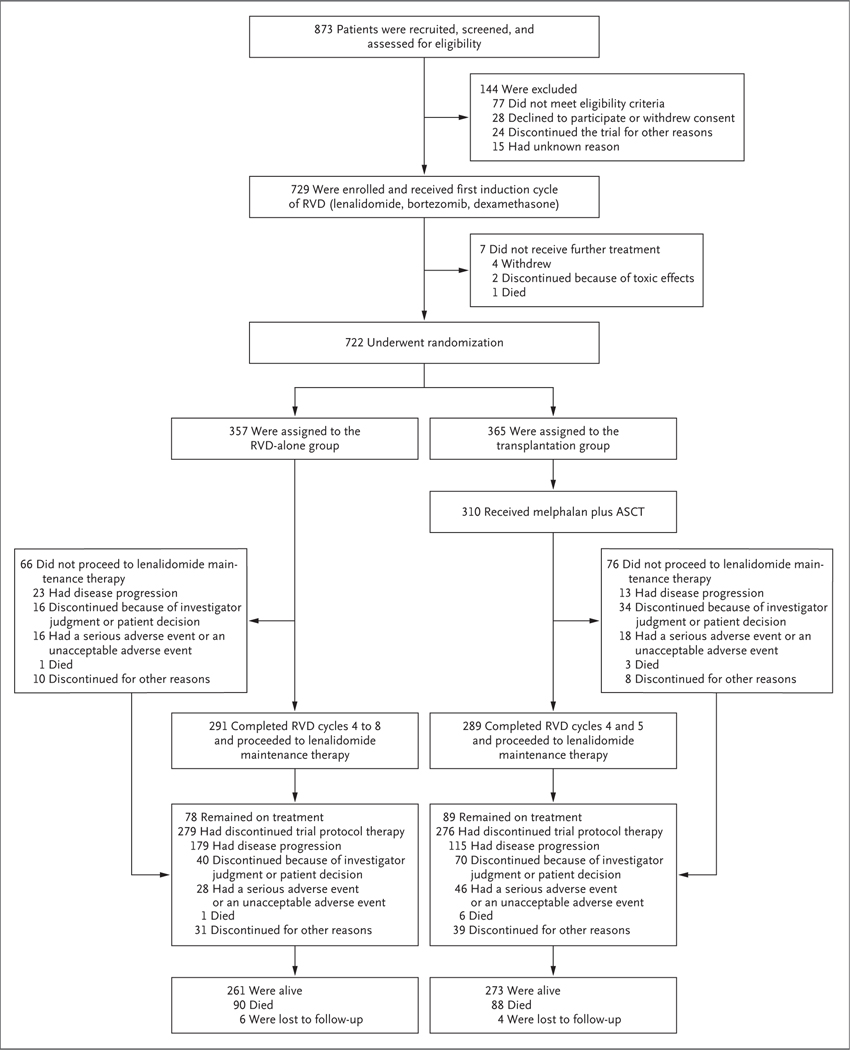
Figure 1. Screening, Randomization, Treatment, and Follow-up.
Of the 77 patients who did not meet eligibility criteria, 32 did not have measurable disease or had minimal measurable disease, 9 did not have end‑organ damage as defined by the CRAB criteria (i.e., hypercalcemia, renal insufficiency, anemia, or bone lesions),13 26 had laboratory values outside permitted cutoff levels, 4 had exceeded the limit of previous therapy, and 6 had screening failure. Of the 24 patients who discontinued the trial for other reasons, 9 had another complicating disease, 8 had insurance issues, 4 discontinued because of physician decision, 2 were unable to adhere to the trial protocol, and 1 had received an alternative therapy. The 76 patients who did not receive lenalidomide maintenance therapy included the 55 patients who had not received melphalan and undergone autologous stem‑cell transplantation (ASCT). Of the 31 patients in the RVD (lenalidomide, bortezomib, dexamethasone)–alone group who discontinued the trial therapy for other reasons, 10 (2 before maintenance therapy) had received therapy outside the trial protocol for another cancer, 2 (1 before maintenance therapy) had received therapy outside the trial protocol for multiple myeloma, 4 (2 before maintenance therapy) had a treatment delay of more than 6 weeks, 7 (4 before maintenance therapy) withdrew consent, 1 had other reasons for discontinuation before maintenance therapy, and 7 had missing data. Of the 39 patients in the transplantation group who discontinued the trial therapy for other reasons, 13 (1 before maintenance therapy) had received therapy outside the trial protocol for another cancer, 2 (1 before maintenance therapy) had received therapy outside the trial protocol for multiple myeloma, 15 (4 before maintenance therapy) had a treatment delay of more than 6 weeks, 5 (2 before maintenance therapy) withdrew consent, and 4 had missing data.