Abstract
Immune-mediated hepatitis is marked by liver inflammation characterized by immune cell infiltration, chemokine/cytokine production, and hepatocyte injury. C-X3C motif receptor 1 (CX3CR1), as the receptor of chemokine C-X3C motif ligand 1 (CX3CL1)/fractalkine, is mainly expressed on immune cells including monocytes and T cells. Previous studies have shown that CX3CR1 protects against liver fibrosis, but the exact role of CX3CL1/CX3CR1 in acute immune-mediated hepatitis remains unknown. Here, we investigate the role of the CX3CL1/CX3CR1 axis in immune-mediated hepatitis using concanavalin A (ConA)-induced liver injury model in CX3CR1-deficient (Cx3cr1−/−) mice. We observed that Cx3cr1−/− mice had severe liver injury and increased pro-inflammatory cytokines (tumor necrosis factor-alpha [TNF-α], interferon-gamma [IFN-γ], interleukin-1 beta [IL-1β], and IL-6) in serum and liver compared to wild-type (Cx3cr1+/+) mice after ConA injection. The deficiency of CX3CR1 did not affect ConA-induced immune cell infiltration in liver but led to elevated production of TNF-α in macrophages as well as IFN-γ in T cells after ConA treatment. On the contrary, exogenous CX3CL1 attenuated ConA-induced cytokine production in wild type, but not CX3CR1-deficient macrophages and T cells. Furthermore, in vitro results showed that CX3CR1 deficiency promoted the pro-inflammatory cytokine expression by increasing the phosphorylation of nuclear factor kappa B (NF-κB) p65 (p-NF-κB p65). Finally, pre-treatment of p-NF-κB p65 inhibitor, resveratrol, attenuated ConA-induced liver injury and inflammatory responses, especially in Cx3cr1−/− mice. In conclusion, our data show that the deficiency of CX3CR1 promotes pro-inflammatory cytokine production in macrophages and T cells by enhancing the phosphorylation of NF-κB p65, which exacerbates liver injury in ConA-induced hepatitis.
Keywords: CX3CR1, immune-mediated hepatitis, macrophage, T cell, NF-κB p65
Impact Statement
This study aims to examine the role of the CX3CL1/CX3CR1 axis in immune-mediated acute hepatitis. Using Cx3cr1−/− and Cx3cr+/+ mice, we demonstrate that CX3CR1 deficiency aggravated concanavalin A-induced acute liver injury with upregulated pro-inflammatory factors production, but not immune cell infiltration. Our in vitro study showed that CX3CR1 deficiency led to increased NF-κB activation, as well as TNF-α and IFN-γ production in bone marrow–derived macrophages and spleen T cells, respectively. Our data emphasize the important role of CX3CR1 in regulating immune cell function, and demonstrate that the CX3CL1/CX3CR1 axis protects the liver against acute injury, and this further extends our understanding of the CX3CL1/CX3CR1 axis in liver disease. Our study also suggests that CX3CL1/CX3CR1 axis may be a potential therapeutic target in immune-mediated acute hepatitis.
Introduction
Immune-mediated hepatitis comprises persistent inflammatory liver damage, caused by the continuous recruitment and infiltration of injury-related effector T cells in the liver. Viral hepatitis and autoimmunity hepatitis (AHI) are the most common forms of immune-mediated hepatitis. 1 Chemokine-directed immune cell infiltration plays an essential role in the pathogenesis of immune-mediated hepatic injury. 2 The chemokine C-X3C motif ligand 1 (CX3CL1) and its sole receptor, C-X3C motif receptor 1 (CX3CR1), are involved in the recruitment of monocytes/macrophages and T cells and play critical roles in many diseases.3 –5 In patients with severe acute hepatic B hepatitis, the expression of CX3CL1 and CX3CR1 are upregulated. 6 However, the role of the CX3CL1/CX3CR1 axis in immune-mediated hepatic injury remains unclear.
Ample evidence suggests that macrophages and T cells play key roles in liver diseases.7,8 Hepatic macrophages, consisting of fetal-derived resident macrophages, Kupffer cells, and monocyte-derived macrophages, constitute the largest immune cell population in the liver. 9 As the resident macrophages, Kupffer cells are essential for preventing tissue damage and initiating immune responses by producing cytokines and chemokines. 10 In reference to various chemokines, peripheral monocytes rapidly infiltrate the inflamed liver and differentiate to macrophages, exhibiting large heterogeneity in multifarious secretion of proinflammatory cytokines, which further cause hepatocellular damage.11,12 For example, hepatic macrophages are the major source of tumor necrosis factor-α (TNF-α), one of the most prominent hepatotoxicity cytokines. 13 In addition, T cells have a crucial role in viral and autoimmune hepatitis by releasing interferon-γ (IFN-γ), contributing to the disease progression.7,13,14
CX3CL1, also known as fractalkine, is the only known member of the CX3C chemokine family. CX3CL1 exists in two forms: a membrane-bound form and a soluble form. 15 The membrane-bound CX3CL1, containing a chemokine domain, a mucin-like stalk and a transmembrane domain, 16 mediates cell adhesion and signaling transmissions via binding to its sole receptor, CX3CR1, which express on the surface of monocytes/macrophages, dendritic cells, T cells, and natural killer (NK) cells. Membrane-bound CX3CL1 can be cleaved by metalloproteases including a disintegrin and metalloproteinase10 or 17 (ADAM10 or ADAM17), to generate a soluble form of CX3CL1. 17 The soluble CX3CL1 acts as a potent chemoattractant that recruits CX3CR1+ monocytes and T cells.18,19 Although numerous studies have shown that CX3CL1/CX3CR1 signaling plays an essential role in brain, lung, kidney, and cardiovascular diseases, 20 there is little information on the role of CX3CL1/ CX3CR1 axis in acute immune-mediated hepatitis. Previous studies showed that the expression level of CX3CL1 is upregulated in primary biliary cirrhosis, 21 chronic liver injury, and fibrosis. 6 CX3CR1 deficiency in mice promoted liver inflammation and subsequent fibrosis in a carbon tetrachloride (CCl4)-induced liver injury model, 22 as well as in a bile duct ligation–induced fibrosis model. 23 However, the precise role of the CX3CL1/CX3CR1 axis in other liver diseases, especially in acute immune-mediated hepatitis, remains unknown.
The intravenous injection of concanavalin A (ConA) in mice is a widely used murine model of human acute hepatitis.24,25 Here, we investigate the role of CX3CR1 in acute immune-mediated hepatitis using a ConA-induced acute hepatitis model in Cx3cr1−/− and Cx3cr1+/+ mice. We demonstrate that the deficiency of CX3CR1 exacerbated ConA-induced liver injury through upregulation of TNF-α and IFN-γ production in macrophages and T cells, respectively, but did not affect immune cell infiltration in the liver. Meanwhile, an in vitro study showed that CX3CR1 deficiency increased nuclear factor kappa B (NF-κB) activation in macrophages and T cells. Finally, we report that p-NF-κB p65 inhibitor, resveratrol, reduced TNF-α/IFN-γ production and attenuated CX3CR1 deficiency–enhanced ConA-induced liver damage.
Materials and methods
Mice
Cx3cr1CreER+/+ (JAX 021160) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and crossed with wild-type C57BL/6 mice to generate Cx3cr1CreER+/− mice. Cx3cr1CreER+/− mice were further crossed with Cx3cr1CreER+/− mice. Homozygous Cx3cr1CreER+/+ mice are CX3CR1-deficient (Cx3cr1−/−) and littermate Cx3cr1CreER−/− mice are CX3CR1-sufficient (Cx3cr1+/+). All mice had a C57BL/6 background. Both male and female mice were used. All animal experiments were reviewed and approved prior to commencement of the activity by the Animal Care and Use Committee from Tulane University (permit number 633). Mice were kept in the specific-pathogen-free animal facility of Tulane University School of Medicine, with a 12 h light/dark cycle.
Animal model of ConA-induced acute liver inflammation
ConA-induced liver injury in mice is a well-established model to investigate the pathology of human acute hepatitis.26,27 ConA is mainly deposited in the liver after intravenous injection, leading to the activation of T cells and macrophages. The proinflammatory cytokines released from T cells and macrophages will further induce the death of sinusoidal endothelial cells and hepatocytes. This process shares many similarities to human immune-mediated hepatitis. 27 Hence, we chose a ConA-mediated mouse model system to explore the role of CX3CL1/CX3CR1 in acute immune-mediated hepatitis. The type IV concanavalin A (ConA, Sigma, C2010) was dissolved in sterile phosphate-buffered saline (DPBS, Thermo Fisher, 21-031-CV) at 1.8 mg/mL prior to use. For the acute hepatitis model, mice were injected with ConA intravenously at a single dose of 15 mg/kg body weight. All mice were euthanized under CO2 at the endpoint. Serum was obtained at 8 and 24 h after ConA injection for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) measurement.
Histology and immunohistochemistry
Liver specimens were excised and fixed in 4% paraformaldehyde overnight, and embedded in paraffin. Sections of 4 µm thickness were stained with hematoxylin and eosin (H&E) using Histology Research Core Lab. Hepatocyte necrosis was observed by light microscopy.
Immunostaining of macrophages and T cells
After overnight fixation in 4% paraformaldehyde, liver specimens were immersed in 30% sucrose for 24 h, snap frozen in optimal cutting temperature compound (OCT, Thermo Fisher, 50-363-579) and sectioned (7 µm). For macrophage staining, slides were immunostained with rat anti-F4/80 antibody (1:400 dilution, Bio-rad, MCA497GA) overnight at 4°C, and visualized with Alexa Fluor 594-conjugated donkey anti-rat IgG antibody (1:500 dilution, Invitrogen, A-21209). For T cell staining, slides were immunostained with Alexa Fluor 488-conjugated rabbit anti-CD3 antibody (1:200 dilution, Santa Cruz, sc-20047) overnight at 4°C. Images were assessed and acquired on a Leica DMRE Research Epi-Fluorescence microscope.
In vitro stimulation of bone marrow–derived macrophages
Bone marrow–derived macrophages (BMDMs) from Cx3cr1+/+ and Cx3cr1−/− mice were digested with a gentle cell dissociation reagent (Gibco, A11105-01), and incubated with 10 μg/mL ConA in dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 20 ng colony-stimulating factor-1 (CSF-1) at 37°C.
Spleen cells and T cells
Spleens were excised from euthanized Cx3cr1+/+ and Cx3cr1−/− mice. Spleens were mashed with plungers of 5 mL syringe in phosphate-buffered saline (PBS) and passed through 40 μm cell strainers. Spleen cells were then resuspended in 1× red blood cell (RBC) lysis buffer (Biolegend, 420301) to remove red blood cells, and kept on ice after washing with PBS. Splenic T cells and non-T cells were isolated using MACS Pan T Cell isolation kit (Miltenyi Biotec, 130-095-130) following the manufacturer’s protocol. Spleen cells, T cells and non-T cells were plated and incubated with 10 μg/mL ConA in DMEM supplemented with 10% FBS, respectively.
Flow cytometry
The fresh liver tissues were harvested from euthanized Cx3cr1+/+ and Cx3cr1−/− mice after ConA injection. Livers were mashed and passed through 70µm cell strainers to get single cell suspension. After washing twice with PBS, the pellet was resuspended in 10 mL 40% Percoll and centrifuged at 500 g for 25 min. After centrifugation, the pellet containing hepatic immune cells was resuspended in flow cytometry staining buffer (PBS with 2% bovine serum albumin [BSA]). The immune cells suspension was washed twice with staining buffer and incubated with CD16/32 (FcγRIII/II, ebioscience, 101325) at 4°C for 15 min to block non-specific Fc receptor binding. The antibody cocktails were added and incubated at 4°C for 30 min. The antibodies used are listed in Supplemental Table1. Samples were analyzed on a BD FACSAria flow cytometer and analyzed using Flow-Jo software.
Western blot
Total protein from BMDM and T cells was extracted using the M-PER Protein Extraction Reagent (Pierce, Rockford, IL) containing protease and phosphatase inhibitors according to the manufacturer’s protocol. Protein concentration was determined using the bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, 23225). Total 30–60 μg total protein was loaded and separated on 4–15% protein gels (Bio-Rad, 456-8084), transferred onto polyvinylidene difluoride (PVDF) membrane (Bio-Rad, 1620177) and blocked with 2% BSA in Tris-buffered saline with Tween-20 (TBST) for 1 h. Membranes were then incubated with the following primary antibodies: NF-κB p65 (Cell Signaling, 8242S), P-NF-κB p65 (Cell Signaling, 3033S), and β-actin (Cell Signaling, 4970S), overnight at 4°C. All membranes were washed three times with TBST (10 mM Tris-HCl, 150 mM NaCl, and 0.1% Tween-20), and then incubated with a secondary goat anti-rabbit antibody for 1 h at room temperature. The signals were visualized using ECL Western Blotting Substrate (Thermo Scientific, 32109). Band density was quantified using Image J software.
Cytokine/chemokine assessment
The mouse serum cytokines were measured using the LEGENDplexTM mouse inflammation kit (Biolegend, 740446), including interleukin-1 alpha (IL-1α), IL-1β, IL-6, IL-10, IL-12p70, IL-17A, IL-23, IL-27, MCP-1, IFN-β, IFN-γ, TNF-α, and GM-CSF. Cytokines (TNF-α, IFN-γ, IL-6, IL-1β, and MCP-1) in the cell culture supernatant and serum were measured using ELISA kits (R&D Systems)
Quantitative real-time polymerase chain reaction
Total RNA was extracted from frozen liver tissues using TRIzol reagent (Invitrogen, 15596018), and purified by RNeasy Mini kit (Qiagen, 74104). Total RNA (500 ng) was converted to complementary DNA (cDNA) using a high-capacity cDNA reverse transcription kit (Applied Biosystems, 4374967). Equal amounts of cDNA were added to SYBR Green Master Mix (Applied Biosystems, A25742) to detect target transcripts expression. Data for relative expression were calculated using the 2−∆∆Ct method. Primers used for quantitative real-time polymerase chain reaction (qPCR) are listed as follows: IFN-γ: forward 5′-CAGCAACAGCAAGGCGAAAAAGG-3′, reverse 5′-TTTCCGCTTCCTGAGGCTGGAT-3′; TNF-α: forward 5′-CCCTCACACTCAGATCATCTTCT-3′, reverse 5′-GCTACGACGTGGGCTACAG-3′; IL-6: forward 5′-TAGTCCTTCCTACCCCAATTTCC-3′, reverse 5′-TTGGTCCTTAGCCACTCCTTC-3′; IL-1β: forward 5′-GCAACTGTTCCTGAACTCAACT-3′, reverse 5′-ATCTTTTGGGGTCCGTCAACT-3′; MCP1: forward 5′-ACACCCTGTTTCGCTGTAGG-3′, reverse 5′-GATTCCTGGAAGGTGGTCAA-3′; CX3CL1: forward 5′-ACGAAATGCGAAATCATGTGC-3′, reverse 5′-CTGTGTCGTCTCCAGGACAA-3′; GAPDH, forward 5′-AGGTCGGTGTGAACGGATTTG-3′, reverse 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Statistical analysis
Data are presented as mean ± SEM. Statistical analysis was performed using GraphPad Prism. Differences between groups were analyzed by unpaired Student’s t-test. A p-value less than 0.05 was taken to denote statistical significance.
Results
Increased ConA-induced liver injury in Cx3cr1−/− mice compared to wild-type Cx3cr1+/+ mice
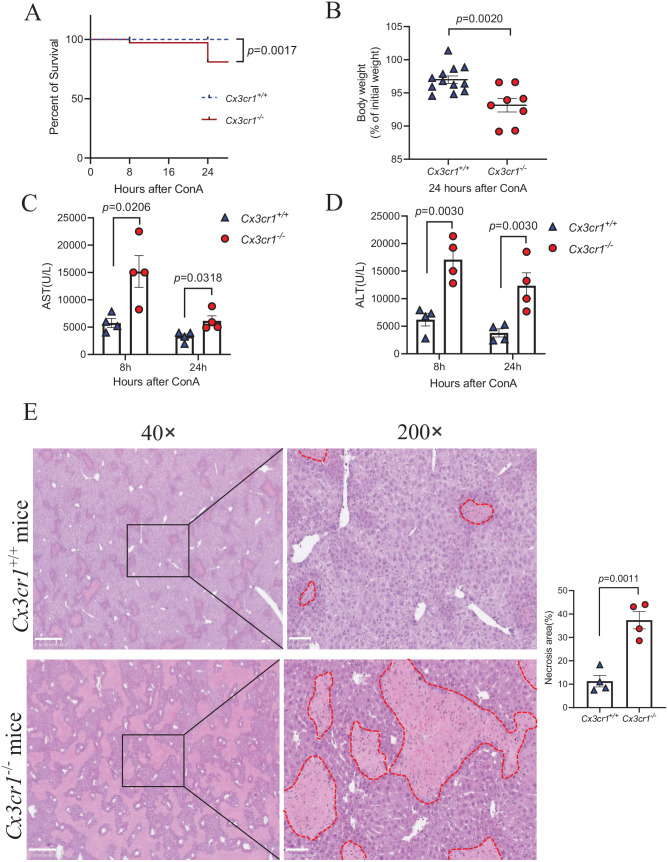
To investigate whether the CX3CL1/CX3CR1 axis is involved in the pathogenesis of immune-mediated hepatitis, we first established the ConA-induced liver injury mouse model by a single-dose ConA injection in mice. We observed that ConA injection led to increased CX3CL1 levels in the liver and serum of wild-type mice (Supplemental Figure 1). Next, we injected ConA into Cx3cr1−/− and Cx3cr1+/+ mice and found that Cx3cr1−/− mice had a significantly higher mortality rate than age- and sex-matched Cx3cr1+/+ mice at 24 h after ConA injection (Figure 1(A)). Furthermore, the surviving Cx3cr1−/− mice had more body weight loss than Cx3cr1+/+ mice (Figure 1(B)). To evaluate ConA-induced liver injury, we measured AST and ALT levels, which are markers of liver injury. As illustrated in Figure 1(C) and (D), Cx3cr1−/− mice had significantly higher serum AST and ALT levels than Cx3cr1+/+ mice at 8 and 24 h after ConA injection. Consistently, H&E staining of liver sections showed an increased hepatocyte necrosis area in Cx3cr1−/− mice compared to Cx3cr1+/+ mice (Figure 1(E)). Taken together, these results demonstrate that the absence of CX3CR1 signaling exacerbates ConA-induced immune-mediated hepatitis.
Figure 1.
Increased Con A-induced liver injury in Cx3cr1−/− mice compared to wild-type Cx3cr1+/+ mice.
Immune-mediated hepatitis was induced by intravenous injection with 15 mg/kg body weight of ConA in Cx3cr1+/+ and Cx3cr1−/− mice. (A) Kaplan-Meier survival curves of Cx3cr1+/+ (n = 12) and Cx3cr1−/− (n = 12) mice. (B) Cx3cr1+/+ (n = 12) and Cx3cr1−/− (n = 8) mice were weighed before ConA injection (initial body weight), and before euthanasia (final body weight). The data of A and B were combined from three independent experiments. (C, D) Serum AST and ALT were measured at 8 and 24 h after ConA treatment (Cx3cr1+/+ n = 4; Cx3cr1−/− n = 4). (E) Left: Representative H&E staining in the liver of ConA-treated Cx3cr1+/+ mice and Cx3cr1−/− mice. (original magnification: 40× and 200×). The scale bars are 500 μm (40×) and 100 μm (200×), respectively. Right: The quantification of liver necrosis area. (A color version of this figure is available in the online journal.)
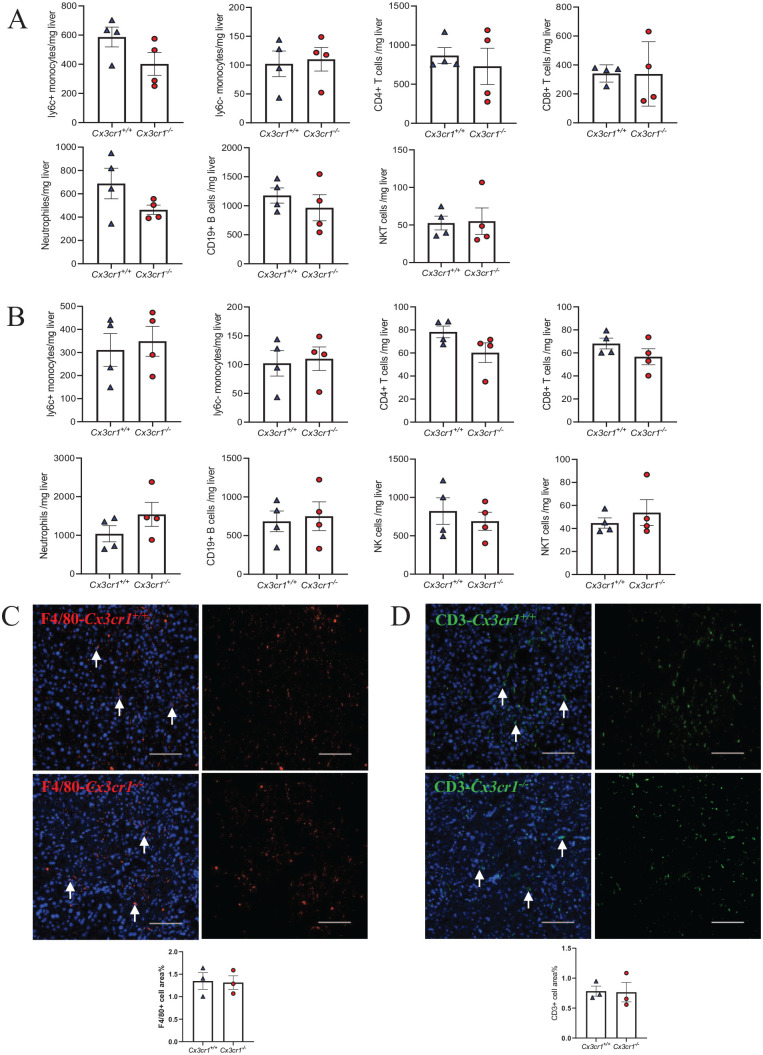
CX3CR1 deficiency does not affect ConA-induced immune cell infiltration in liver
To investigate the underlying mechanism of increased liver injury in CX3CR1-deficient mice, we examined the number of various immune cells that infiltrated into the liver of Cx3cr1−/− and Cx3cr1+/+ mice at 8 and 14 h after ConA injection. We chose these two time points because the liver injury reached the peak severity at 8 h and began to subside at 24 h after ConA injection. Unexpectedly, we did not observe a significant difference in the cell counts, including ly6C + monocytes, ly6C- monocytes, CD4+ T cells, CD8+ T cells, and NKT cells, between Cx3cr1−/− and Cx3cr1+/+ mice at both time points (Figure 2(A) and (B)). Since macrophages and T cells play essential roles in ConA-induced inflammation, 28 we examined the distribution of hepatic macrophages and T cells by immunofluorescent (IF) staining using specific antibodies against F4/80 and CD3, markers of macrophage and T cell, respectively. Consistent with the fluorescence activated cell sorting (FACS) results, we did not observe a significant difference in macrophage or T cell density in the liver section obtained 24 h after ConA injection between Cx3cr1+/+ and Cx3cr1−/− mice (Figure 2(C) and (D)). These data indicate that the absence of CX3CR1 does not contribute to liver injury through altering the recruitment of immune cells.
Figure 2.
CX3CR1 deficiency does not affect ConA-induced immune cells infiltration in liver.
(A, B) ConA-induced immune cell infiltration in the liver of Cx3cr1−/− (n = 4) and Cx3cr1+/+ (n = 4) mice were analyzed by flow cytometry. Liver samples were collected at 8 h (A) and 14 h (B) after ConA treatment. (C, D) Representative immunofluorescence staining of F4/80 (C) and CD3 (D) and quantification in the liver of Cx3cr1−/− and Cx3cr1+/+ mice at 24 h after ConA (The scale bars are 100 μm). (A color version of this figure is available in the online journal.)
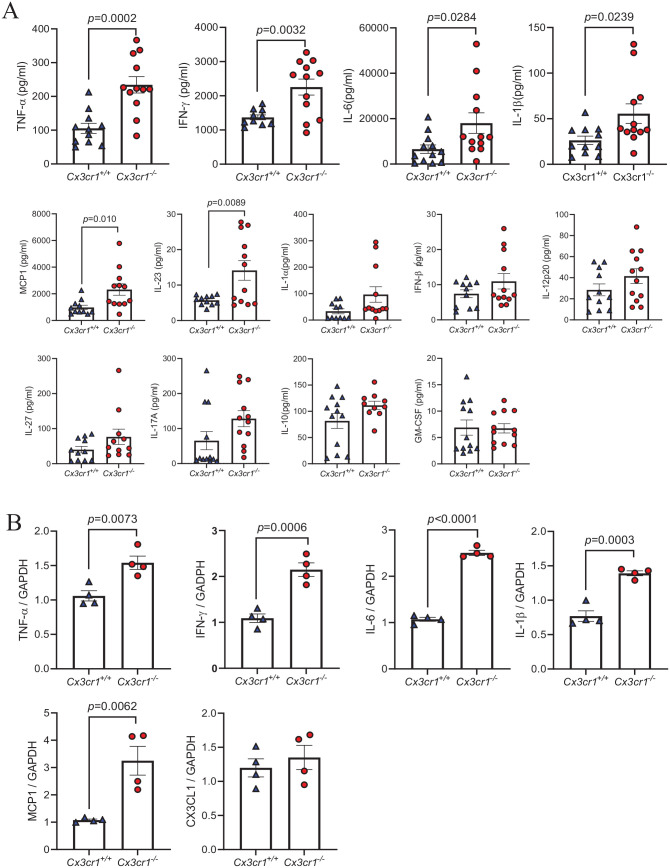
CX3CR1 deficiency leads to increased cytokine and chemokine levels in serum and liver
Since CX3CR1 deficiency does not affect the immune cell recruitment, we next investigated whether it would affect the functionality of immune cells, with the focus on cytokine and chemokine release, which play an important role in ConA-induced hepatitis. We first measured the serum cytokine and chemokine level at 8 and 24 h after ConA injection and found that, compared to Cx3cr1+/+ mice, many pro-inflammatory cytokines and chemokines exhibited an upward tendency in Cx3cr1−/− mice (Figure 3(A)). Among them, TNF-α, IFN-γ, IL-6, IL-1β, and MCP-1 are significantly increased in Cx3cr1−/− mice (Figure 3(A)). Next, we measured the mRNA level of these cytokines and chemokines in the liver of Cx3cr1+/+ and Cx3cr1−/− mice at 3 h after ConA injection. We found that hepatic proinflammatory gene expression including TNF-α, IL-1β, IL-6, IFN-γ and MCP-1 was significantly increased in Cx3cr1−/− mice compared to Cx3cr1+/+ mice (Figure 3(B)). Altogether, these data suggest that CX3CR1 deficiency increases the expression of several important proinflammatory cytokines and chemokines, such as TNF-α and IFN-γ, which may lead to more severe inflammation and liver damage.
Figure 3.
CX3CR1 deficiency leads to increased cytokine and chemokine levels in serum and liver.
(A) Serum was collected from Cx3cr1−/− (n = 12) and Cx3cr1+/+ (n = 12) mice at 8 h after ConA treatment, serum cytokines and chemokines were detected by LEGENDplex™ Mouse Inflammation kit. Data were combined from three independent experiments. (B) Hepatic mRNA expressions of TNF-α, IL-1β, IL-6, IFN-γ, CCL2, and CX3CL1 were measured by real-time PCR. Liver samples were harvested from Cx3cr1−/− (n = 4) and Cx3cr1+/+ (n = 4) mice at 3 h after ConA injection. (A color version of this figure is available in the online journal.)
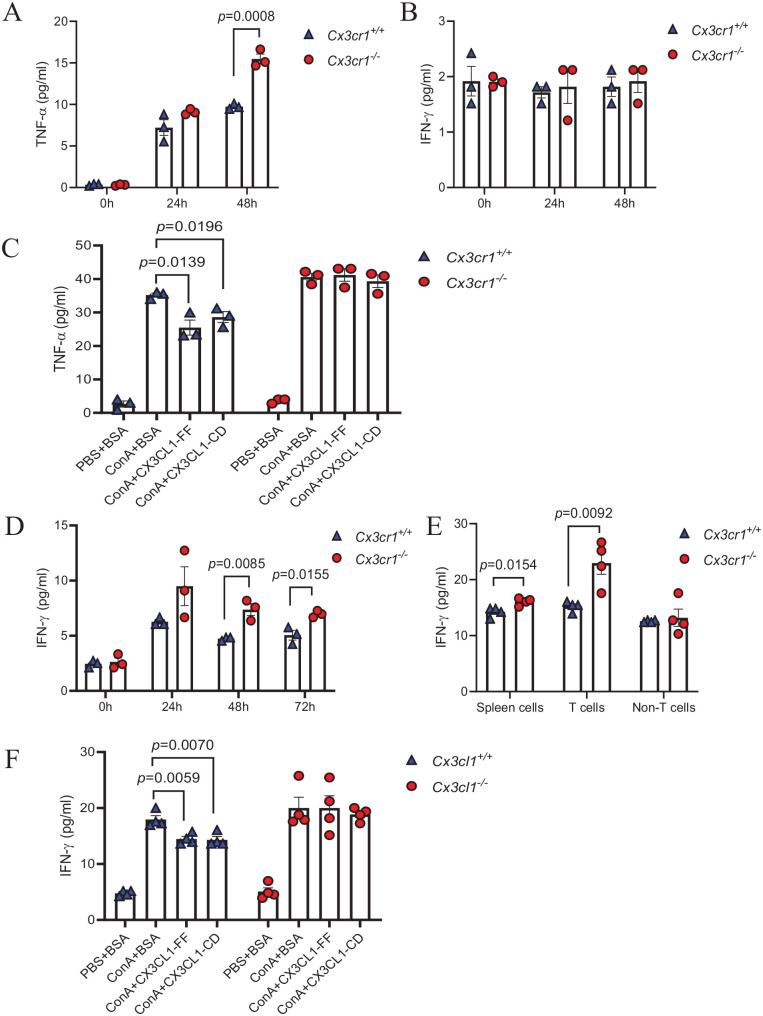
Disruption of CX3CL1/CX3CR1 axis leads to enhanced pro-inflammatory properties of macrophage and T cells
TNF-α and IFN-γ, two main cytokines involved in immune-mediated hepatic injury, can be produced by macrophages and T cells, which also expressed CX3CR1. To investigate whether the absence of CX3CR1 influences the cytokine release from these cells, we compared the TNF-α and IFN-γ production of BMDMs or splenic T cells from Cx3cr1−/− and Cx3cr1+/+ mice. For macrophages, we incubated CX3CR1-deficient or sufficient BMDMs with ConA for 24 and 48 h, followed by measuring TNF-α and IFN-γ in the supernatant. We found that BMDMs from Cx3cr1−/− mice secreted significantly more TNF-α than Cx3cr1+/+ mice at 48 h after ConA treatment (Figure 4(A)), but there was no significant difference in the level of IFN-γ between Cx3cr1−/− and Cx3cr1+/+ BMDMs (Figure 4(B)). For splenic T cells, we first isolated spleen cells from Cx3cr1−/− and Cx3cr1+/+ mice and treated them with ConA for various time points. We found that CX3CR1-deficient spleen cells secreted more IFN-γ than Cx3cr1+/+ cells (Figure 4(D)). TNF-α was undetectable in both cells. To further determine whether T cell contributes to the enhanced IFN-γ expression, we purified T cells from spleen and incubated them with ConA for 48 h. Interestingly, we observed that splenic T cells from Cx3cr1−/− mice secrete more IFN-γ than that from Cx3cr1+/+ mice (Figure 4(E)). These results suggest that CX3CR1 may act as a negative regulator of cytokine expression. To investigate whether this effect is mediated via the interaction with CX3CL1, we pre-treated BMDMs and splenic T cells with full-length CX3CL1 (CX3CL1-FF) or the chemokine domain of CX3CL1 (CX3CL1-CD), followed by ConA stimulation. We found that exogenous CX3CL1 significantly inhibited ConA-induced TNF-α expression in Cx3cr1+/+ BMDMs (Figure 4(C)), as well as IFN-γ expression in Cx3cr1+/+ T cells (Figure 4(D)). However, there was no effect in Cx3cr1−/− BMDMs and T cells. These results suggest that the CX3CL1/CX3CR1 axis is a negative regulator of ConA-induced cytokine production, and interruption of this axis leads to enhanced pro-inflammatory properties of macrophages and T cells.
Figure 4.
Disruption of CX3CL1/CX3CR1 axis leads to enhanced pro-inflammatory properties of macrophages and T cells.
(A, B) TNF-α and IFN-γ levels in the supernatant of ConA-treated Cx3cr1+/+ and Cx3cr1−/− BMDMs (10 μg/mL). (C) BMDMs were pretreated with 100 ng/mL full-length CX3CL1 (CX3CL1-FF), 100 ng/mL the domain of CX3CL1 (CX3CL1-CD) or 100 ng/mL BSA, then incubated with ConA (10 μg/mL) for 48 h to detect TNF-α level. (D) IFN-γ levels of ConA-treated spleen cells (10 μg/mL) were detected by ELISA assay. (E) T cells and non-T cells in spleen cells were separated by pan T cells isolation kit, then total spleen cells, T cells, and non-T cells incubated with ConA for 48 h. IFN-γ levels of each group were measured by ELISA assay. (F) IFN-γ levels of the supernatant of T cells were detected after co-incubation with ConA + BSA, ConA + CX3CL1-CD and ConA + CX3CL1-FF. (A color version of this figure is available in the online journal.)
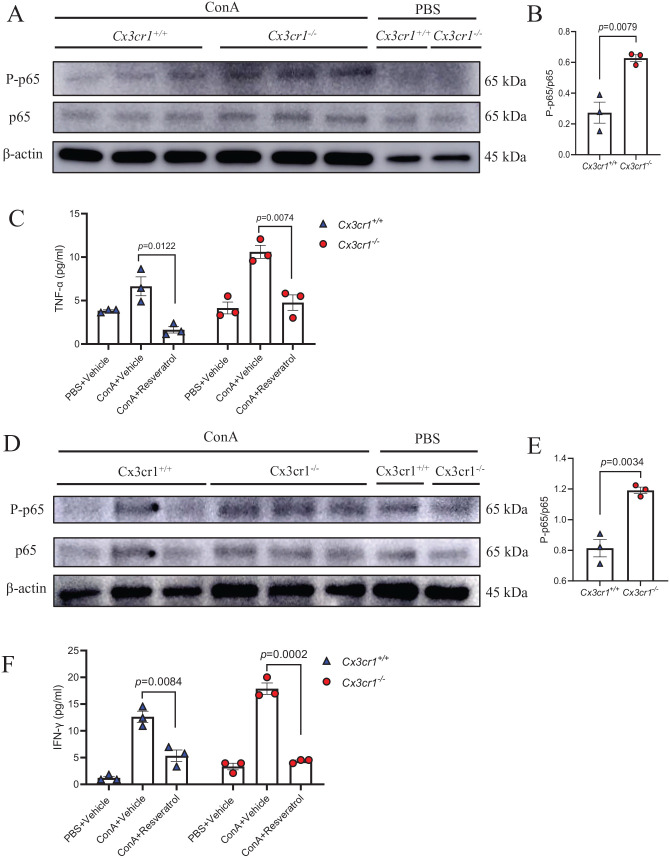
The deficiency of CX3CR1 increased the inflammation by increasing the phosphorylation of NF-κB-p65 in macrophages and T cells
To further investigate the mechanism underlying CX3CR1 deficiency–increased cytokine production, we determined the activation of NF-κB pathway, a well-established inducer of pro-inflammatory cytokines, in BMDMs and T cells from Cx3cr1−/− and Cx3cr1+/+ mice. To this end, we incubated Cx3cr1−/− and Cx3cr1+/+ BMDMs with ConA for 48 h before protein extraction. As indicated in Figure 5(A) and (B), the phosphorylation of NF-κB-p65 was higher in Cx3cr1−/− BMDMs than Cx3cr1+/+ BMDMs. Furthermore, we treated Cx3cr1−/− and Cx3cr1+/+ BMDMs with ConA and resveratrol, an inhibitor of NF-κB p65 phosphorylation. We found that the TNF-α expression in BMDMs were significantly inhibited, especially in Cx3cr1−/− BMDMs (Figure 5(C)). We also observed a similar phenomenon in T cells, which showed increased NF-κB-p65 phosphorylation in Cx3cr1−/− T cells. Importantly, resveratrol abolished increased IFN-γ expression in Cx3cr1−/− T cells (Figure 5(D) to (F)). Taken together, these results suggest that the deficiency of CX3CR1 enhanced the proinflammatory properties of macrophages and T cells through active NF-κB-p65 phosphorylation.
Figure 5.
The deficiency of CX3CR1 increased the inflammation by increasing the phosphorylation of NF-κB-p65 in macrophages and T cells.
BMDMs and T cells were isolated from Cx3cr1+/+ and Cx3cr1−/− mice and treated with ConA or PBS. (A and B, D and E) The phosphorylation level of NF-κB-p65 in BMDMs (A) and T cells (D) was detected by Western blotting, their quantification is shown in B and E. (C) BMDMs were incubated with PBS + vehicle (dimethyl sulfoxide), ConA + vehicle and ConA + resveratrol for 48 h, and their TNF-α level was measured by ELISA. (F) T cells were incubated with the same combination and IFN-γ level was measured by ELISA. (A color version of this figure is available in the online journal.)
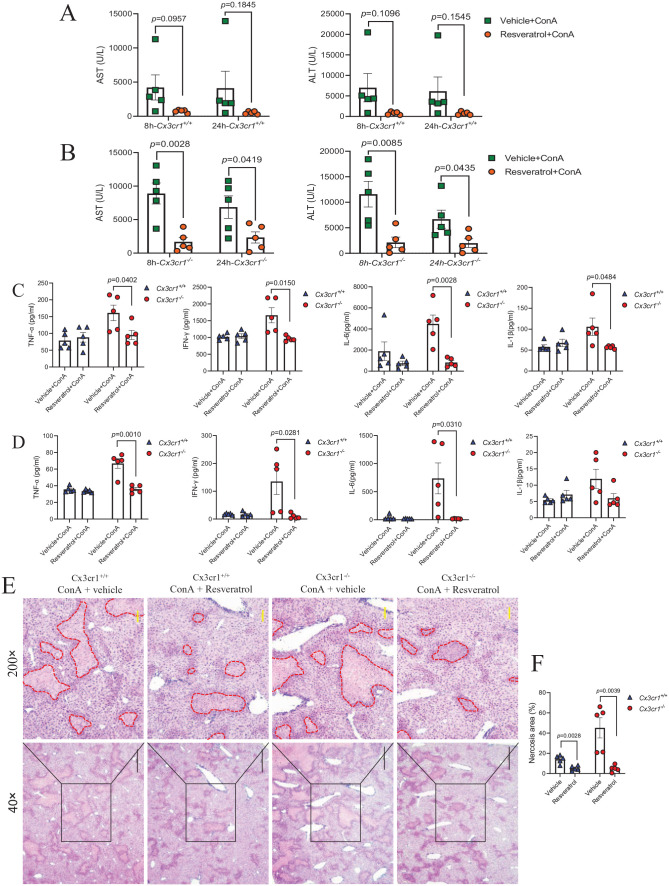
P-NF-κB p65 inhibitor resveratrol can protect the liver from CX3CR1 deficiency–enhanced ConA-induced damage
Furthermore, we examined the effect of resveratrol in ConA-induced liver injury in Cx3cr1−/− and Cx3cr1+/+ mice. To this end, we treated mice with resveratrol or control vehicle by oral gavage for seven consecutive days, and injected mice with ConA after the last gavage. In contrast to Cx3cr1+/+ mice, which only showed a downward trend of serum AST and ALT after resveratrol treatment (Figure 6(A)), the pretreatment of resveratrol in Cx3cr1−/− mice significantly attenuated ConA-induced liver injury, as evidenced by the reduction of serum AST and ALT levels at 8 or 24 h after ConA injection (Figure 6(B)). H&E staining showed that resveratrol treatment significantly attenuated the ConA-induced hepatocyte necrosis area in both Cx3cr1−/− and Cx3cr1+/+ mice (Figure 6(E) and (F)). To further determine the effect of resveratrol on the ConA-induced cytokine production, we compared the proinflammatory level in the serum of Cx3cr1−/− and Cx3cr1+/+ mice at 8 and 24 h after ConA injection. We found that resveratrol specifically reduced the level of serum cytokines, including TNF-α, IFN-γ, IL-6, and IL-1β in Cx3cr1−/− mice, but not Cx3cr1+/+ mice, at both time points (Figure 6(C) and (D)). Taken together, these results demonstrate that inhibition of NF-κB-p65 phosphorylation can protect the liver from the enhanced damage due to the deficiency of CX3CR1.
Figure 6.
P-NF-κB-p65 inhibitor resveratrol can protect the liver from CX3CR1 deficiency–enhanced ConA-induced damage.
Cx3cr1+/+ and Cx3cr1−/− mice were pre-treated with 30 mg/kg body weight of resveratrol or vehicle (DMSO) seven times by intragastric administration, followed by intravenous injection with 13 mg/kg body weight of ConA. (A, B) Serum AST and ALT were measured at 8 and 24 h after ConA injection. (C, D) Serum TNF-α, IFN-γ, IL-6, IL-1β levels at 8 h (C) and 24 h (D) after ConA injection. (E) Liver necrosis was evaluated by H&E staining (original magnification: 40× and 200×). The scale bars are 500 μm (40×) and 100 μm (200×), respectively. (F) The quantification of liver necrosis area. (A color version of this figure is available in the online journal.)
Discussion
In this study, we demonstrate that the deficiency of CX3CR1 ameliorates ConA-induced immune-mediated hepatitis and proinflammatory cytokine production. Interestingly, we observed that CX3CR1 deficiency did not affect immune cell infiltration in the liver but led to increased expression of TNF-α production in BMDMs, and IFN-γ production in splenic T cells, which were associated with the enhanced NF-κB-p65 phosphorylation, an indicator of NF-kB activation. Finally, the inhibition of NF-κB-p65 phosphorylation attenuated ConA-induced cytokine release and liver injury.
ConA-induced hepatitis is a well-established experimental murine model of acute immune-mediated hepatitis. In contrast to other models, ConA-induced liver injury is primarily driven by the activation of T cells and macrophages and the production of inflammatory cytokines, which lead to hepatocyte damage. These features resemble the pathophysiological process of viral or autoimmune hepatitis in humans.25,29 Utilizing this model, we observed that ConA injection led to increased CX3CL1 in mice, which is consistent with a previous finding in human viral hepatitis, 6 further confirming its feasibility as an immune-mediated hepatitis model. Numerous studies have demonstrated the beneficial or detrimental role of CX3CR1 in diseases, mostly through its interaction with the sole ligand, CX3CL1, mediating leukocyte recruitment.30,31 However, in our study, CX3CR1-deficient and CX3CR1-sufficient mice exhibited similar immune cell infiltration in the liver after ConA, which suggests that CX3CL1-induced chemotaxis is tissue specific. Indeed, we previously demonstrated that CX3CL1/CX3CR1-mediated monocyte recruitment is specifically required for kidney macrophage regeneration, but not for macrophages in the lung and spleen. 18 Consistently, in a CCl4-induced liver damage model, CX3CR1 deficiency did not change the number of leukocytes and macrophages in 48 h after CCl4, but prolonged macrophage survival. 23
We also demonstrate that CX3CR1 deficiency leads to increased TNF-α production in bone marrow–derived macrophages. Many studies have shown that CX3CR1 not only acts as a chemokine receptor, but also can initiate intracellular signaling pathways upon binding with CX3CL1. 32 A previous study also showed that Kupffer cells in CX3CR1-deficient mice had increased expression of TNF-α and TGF-β in a CCl4-induced chronic liver injury/fibrosis model. 22 It should be noted that CX3CR1 mainly expressed on monocyte-derived macrophages, rather than steady-state resident Kupffer cells in the liver.33,34 Further study is required to determine the relative effect of CX3CR1 deficiency on either monocyte-derived macrophages or resident Kupffer cells. CX3CR1 was reported to control the differentiation and survival of infiltrating monocytes in liver fibrosis. 23 Although we did not notice a difference in infiltrated ly6C + or ly6C- monocytes between Cx3cr1−/− and Cx3cr1+/+ mice after ConA, it will be interesting to determine the effect of CX3CR1 on macrophage polarization (M1 versus M2) in further studies.
In this study, we found that CX3CR1 deficiency increased ConA-induced IFN-γ expression in splenic T cells. A previous study has demonstrated that CX3CR1 is expressed in hepatic T cells and its deficiency impaired the IL-17A production and Th17 cell differentiation. 35 Whether this is the case in our system needs further investigation. Interestingly, supplementary exogenous CX3CL1 reduced ConA-induced cytokine production in both macrophages and splenic T cells, suggesting that CX3CL1 may be a negative regulator of immune activation in immune-mediated hepatitis. Our results showed that CX3CR1 deficiency led to increased NF-κB-p65 phosphorylation, and inhibition of NF-κB activation protected the liver from CX3CR1 deficiency-enhanced ConA-induced damage. Previous studies also showed that the CX3CL1/CX3CR1 axis is involved in inflammatory processes through several inflammatory signaling pathways including NF-κB, 32 a well-known inducer of the expression of various pro-inflammatory genes. For example, CX3CL1 promoted the transportation of p65 protein out of the nucleus and led to the reduction of TNF-α in RAW264.7 cells, which demonstrates that CX3CL1 participated in rendering intestine resident macrophages to become hyporesponsive to LPS. 36 In another study, Cx3cr1−/− mice exhibited increased phosphorylation of p65 in a model of amyotrophic lateral sclerosis, 37 and acetylation of p65 in microglia of dentate gyrus. 38 However, in an LPS-induced lung injury model, Cx3cr1−/− mice exhibited reduced NF-κB activation compared to WT mice. 39 Thus, the CX3CL1/CX3CR1/ NF-κB axis may play opposite roles in different diseases, depending on target cells and disease progression. It should be noted that although resveratrol has been demonstrated to suppress NF-κB-dependent cytokine production,40 –42 it also affects other signaling pathways, 43 which may contribute to the reduced liver injury without influencing the production of TNF-α and IFN-γ. Further study is required to investigate the effect of resveratrol in these pathways. Our in vitro data demonstrate that CX3CL1 inhibited pro-inflammatory cytokine expression both in macrophages and T cells, which suggests that the effect of CX3CL1 is not restricted to myeloid cells. Although we did not determine the effect of CX3CL1 in vivo in this study, other studies have demonstrated the beneficial effect of recombinant CX3CL1 44 or CX3CL1-Fc 45 treatment in disease models.
In conclusion, we demonstrate that the deficiency of CX3CR1 led to increased NF-κB activation, which upregulated pro-inflammatory cytokine production in macrophages and T cells, further exacerbating liver injury in ConA-induced acute immune-mediated hepatitis. The interruption of the CX3CL1/CX3CR1/NF-kB axis attenuated liver inflammation and injury. Thus, the CX3CR1/CX3CR1 axis may be a potential therapeutic target in immune-mediated hepatitis.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221128573 for CX3CR1 deficiency exacerbates immune-mediated hepatitis by increasing NF-κB-mediated cytokine production in macrophage and T cell by Mi Ren, Jinyan Zhang, Shen Dai, Chenxiao Wang, Zheng Chen, Siqi Zhang, Junming Xu, Xuebin Qin and Fengming Liu in Experimental Biology and Medicine
Footnotes
Authors’ Contributions: All the authors designed and performed the experiments. MR, JZ, and FL interpreted the results and supervised the experiments. MR, JZ, XQ, and FL supervised the project and analyzed the data. MR, JZ, and FL wrote the article and all authors participated in the review and critique of the article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Tulane start-up funds, the National Natural Science Foundation of China (81670595 and 81970568), Shanghai Shenkang Hospital Development Center Clinical Science and Technology Innovation Project (SHDC12020104), and the Natural Science Foundation of Shandong Province (2022HWYQ-053).
ORCID iD: Fengming Liu  https://orcid.org/0000-0002-8815-3356
https://orcid.org/0000-0002-8815-3356
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Chu H, Wang J, Wang Q, Chen J, Li J, Li H, Zhang L. Protective effect of n-butanol extract from Viola yedoensis on immunological liver injury. Chem Biodivers 2021;18:e2001043 [DOI] [PubMed] [Google Scholar]
- 2. Heymann F, Tacke F. Immunology in the liver–from homeostasis to disease. Nat Rev Gastroenterol Hepatol 2016;13:88–110 [DOI] [PubMed] [Google Scholar]
- 3. Cormican S, Griffin MD. Fractalkine (CX3CL1) and its receptor CX3CR1: a promising therapeutic target in chronic kidney disease? Front Immunol 2021;12:664202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Staumont-Sallé D, Fleury S, Lazzari A, Molendi-Coste O, Hornez N, Lavogiez C, Kanda A, Wartelle J, Fries A, Pennino D, Mionnet C, Prawitt J, Bouchaert E, Delaporte E, Glaichenhaus N, Staels B, Julia V, Dombrowicz D. CX-CL1 (fractalkine) and its receptor CX-CR1 regulate atopic dermatitis by controlling effector T cell retention in inflamed skin. J Exp Med 2014;211:1185–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murphy G, Caplice N, Molloy M. Fractalkine in rheumatoid arthritis: a review to date. Rheumatology 2008;47:1446–51 [DOI] [PubMed] [Google Scholar]
- 6. Efsen E, Grappone C, DeFranco RM, Milani S, Romanelli RG, Bonacchi A, Caligiuri A, Failli P, Annunziato F, Pagliai G, Pinzani M, Laffi G, Gentilini P, Marra F. Up-regulated expression of fractalkine and its receptor CX3CR1 during liver injury in humans. J Hepatol 2002;37:39–47 [DOI] [PubMed] [Google Scholar]
- 7. Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol 2016;16:509–23 [DOI] [PubMed] [Google Scholar]
- 8. Shan Z, Ju C. Hepatic macrophages in liver injury. Front Immunol 2020;11:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol 2017;66:1300–12 [DOI] [PubMed] [Google Scholar]
- 10. Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol 2017;17:306–21 [DOI] [PubMed] [Google Scholar]
- 11. Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 2014;60:1090–6 [DOI] [PubMed] [Google Scholar]
- 12. Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol 2021;18:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, Shakhov AN, Murakami T, Drutskaya LN, Förster I, Clausen BE, Tessarollo L, Ryffel B, Kuprash DV, Nedospasov SA. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity 2005;22:93–104 [DOI] [PubMed] [Google Scholar]
- 14. Zheng C, Yin S, Yang Y, Yu Y, Xie X. CD24 aggravates acute liver injury in autoimmune hepatitis by promoting IFN-γ production by CD4+ T cells. Cell Mol Immunol 2018;15:260–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee YS, Morinaga H, Kim JJ, Lagakos W, Taylor S, Keshwani M, Perkins G, Dong H, Kayali AG, Sweet IR, Olefsky J. The fractalkine/CX3CR1 system regulates β cell function and insulin secretion. Cell 2013;153:413–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Korbecki J, Simińska D, Kojder K, Grochans S, Gutowska I, Chlubek D, Baranowska-Bosiacka I. Fractalkine/CX3CL1 in neoplastic processes. Int J Mol Sci 2020;21:3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Julia V. CX3CL1 in allergic diseases: not just a chemotactic molecule. Allergy 2012;67:1106–10 [DOI] [PubMed] [Google Scholar]
- 18. Liu F, Dai S, Feng D, Qin Z, Peng X, Sakamuri S, Ren M, Huang L, Cheng M, Mohammad KE, Qu P, Chen Y, Zhao C, Zhu F, Liang S, Aktas BH, Yang X, Wang H, Katakam PVG, Busija DW, Fischer T, Datta PK, Rappaport J, Gao B, Qin X. Distinct fate, dynamics and niches of renal macrophages of bone marrow or embryonic origins. Nat Commun 2020;11:2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sans M, Danese S, de la Motte C, de Souza HS, Rivera-Reyes BM, West GA, Phillips M, Katz JA, Fiocchi C. Enhanced recruitment of CX3CR1+ T cells by mucosal endothelial cell-derived fractalkine in inflammatory bowel disease. Gastroenterology 2007;132:139–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee M, Lee Y, Song J, Lee J, Chang SY. Tissue-specific role of CX(3)CR1 expressing immune cells and their relationships with human disease. Immune Netw 2018;18:e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Chemokine-chemokine receptor CCL2-CCR2 and CX3CL1-CX3CR1 axis may play a role in the aggravated inflammation in primary biliary cirrhosis. Dig Dis Sci 2014;59:358–64 [DOI] [PubMed] [Google Scholar]
- 22. Aoyama T, Inokuchi S, Brenner DA, Seki E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 2010;52:1390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karlmark KR, Zimmermann HW, Roderburg C, Gassler N, Wasmuth HE, Luedde T, Trautwein C, Tacke F. The fractalkine receptor CX-CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 2010;52:1769–82 [DOI] [PubMed] [Google Scholar]
- 24. Heymann F, Hamesch K, Weiskirchen R, Tacke F. The concanavalin A model of acute hepatitis in mice. Lab Anim 2015;49:12–20 [DOI] [PubMed] [Google Scholar]
- 25. Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest 1992;90:196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knolle PA, Gerken G, Loser E, Dienes HP, Gantner F, Tiegs G, Meyer zum Buschenfelde KH, Lohse AW. Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology 1996;24:824–9 [DOI] [PubMed] [Google Scholar]
- 27. Wang HX, Liu M, Weng SY, Li JJ, Xie C, He HL, Guan W, Yuan YS, Gao J. Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J Gastroenterol 2012;18:119–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higashimoto M, Sakai Y, Takamura M, Usui S, Nasti A, Yoshida K, Seki A, Komura T, Honda M, Wada T, Furuichi K, Ochiya T, Kaneko S. Adipose tissue derived stromal stem cell therapy in murine ConA-derived hepatitis is dependent on myeloid-lineage and CD4+ T-cell suppression. Eur J Immunol 2013;43:2956–68 [DOI] [PubMed] [Google Scholar]
- 29. Khan HA, Ahmad MZ, Khan JA, Arshad MI. Crosstalk of liver immune cells and cell death mechanisms in different murine models of liver injury and its clinical relevance. Hepatobiliary Pancreat Dis Int 2017;16:245–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brett CA, Carroll JB, Gabriele ML. Compromised fractalkine signaling delays microglial occupancy of emerging modules in the multisensory midbrain. Glia 2022;70:697–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muraoka S, Kaneko K, Motomura K, Nishio J, Nanki T. CX3CL1/fractalkine regulates the differentiation of human peripheral blood monocytes and monocyte-derived dendritic cells into osteoclasts. Cytokine 2021;146:155652. [DOI] [PubMed] [Google Scholar]
- 32. Zhuang Q, Ou J, Zhang S, Ming Y. Crosstalk between the CX3CL1/CX3CR1 axis and inflammatory signaling pathways in tissue injury. Curr Protein Pept Sci 2019;20:844–54 [DOI] [PubMed] [Google Scholar]
- 33. Heymann F, Peusquens J, Ludwig-Portugall I, Kohlhepp M, Ergen C, Niemietz P, Martin C, van Rooijen N, Ochando JC, Randolph GJ, Luedde T, Ginhoux F, Kurts C, Trautwein C, Tacke F. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015;62:279–91 [DOI] [PubMed] [Google Scholar]
- 34. David BA, Rezende RM, Antunes MM, Santos MM, Freitas Lopes MA, Diniz AB, Sousa Pereira RV, Marchesi SC, Alvarenga DM, Nakagaki BN, Araújo AM, Dos Reis DS, Rocha RM, Marques PE, Lee WY, Deniset J, Liew PX, Rubino S, Cox L, Pinho V, Cunha TM, Fernandes GR, Oliveira AG, Teixeira MM, Kubes P, Menezes GB. Combination of mass cytometry and imaging analysis reveals origin, location, and functional repopulation of liver myeloid cells in mice. Gastroenterology 2016;151:1176–91 [DOI] [PubMed] [Google Scholar]
- 35. Dong L, Nordlohne J, Ge S, Hertel B, Melk A, Rong S, Haller H, von Vietinghoff S. T cell CX3CR1 mediates excess atherosclerotic inflammation in renal impairment. J Am Soc Nephrol 2016;27:1753–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mizutani N, Sakurai T, Shibata T, Uchida K, Fujita J, Kawashima R, Kawamura YI, Toyama-Sorimachi N, Imai T, Dohi T. Dose-dependent differential regulation of cytokine secretion from macrophages by fractalkine. J Immunol 2007;179:7478–87 [DOI] [PubMed] [Google Scholar]
- 37. Liu C, Hong K, Chen H, Niu Y, Duan W, Liu Y, Ji Y, Deng B, Li Y, Li Z, Wen D, Li C. Evidence for a protective role of the CX3CL1/CX3CR1 axis in a model of amyotrophic lateral sclerosis. Biol Chem 2019;400:651–61 [DOI] [PubMed] [Google Scholar]
- 38. Sellner S, Paricio-Montesinos R, Spieß A, Masuch A, Erny D, Harsan LA, Elverfeldt DV, Schwabenland M, Biber K, Staszewski O, Lira S, Jung S, Prinz M, Blank T. Microglial CX3CR1 promotes adult neurogenesis by inhibiting Sirt 1/p65 signaling independent of CX3CL1. Acta Neuropathol Commun 2016;4:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ding XM, Pan L, Wang Y, Xu QZ. Baicalin exerts protective effects against lipopolysaccharide-induced acute lung injury by regulating the crosstalk between the CX3CL1-CX3CR1 axis and NF-κB pathway in CX3CL1-knockout mice. Int J Mol Med 2016;37:703–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. He Y, Lu X, Chen T, Yang Y, Zheng J, Chen C, Zhang Y, Lei W. Resveratrol protects against myocardial ischemic injury via the inhibition of NF-κB-dependent inflammation and the enhancement of antioxidant defenses. Int J Mol Med 2021;47:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nallasamy P, Kang ZY, Sun X, Anandh Babu PV, Liu D, Jia Z. Natural compound resveratrol attenuates TNF-alpha-induced vascular dysfunction in mice and human endothelial cells: the involvement of the NF-κB signaling pathway. Int J Mol Sci 2021;22:12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang QB, He YL, Zhong XW, Xie WG, Zhou JG. Resveratrol ameliorates gouty inflammation via upregulation of sirtuin 1 to promote autophagy in gout patients. Inflammopharmacology 2019;27:47–56 [DOI] [PubMed] [Google Scholar]
- 43. Švajger U, Jeras M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int Rev Immunol 2012;31:202–22 [DOI] [PubMed] [Google Scholar]
- 44. Jiang M, Xie H, Zhang C, Wang T, Tian H, Lu L, Xu JY, Xu GT, Liu L, Zhang J. Enhancing fractalkine/CX3CR1 signalling pathway can reduce neuroinflammation by attenuating microglia activation in experimental diabetic retinopathy. J Cell Mol Med 2022;26:1229–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Riopel M, Vassallo M, Ehinger E, Pattison J, Bowden K, Winkels H, Wilson M, de Jong R, Patel S, Balakrishna D, Bilakovics J, Fanjul A, Plonowski A, Larson CJ, Ley K, Cabrales P, Witztum JL, Olefsky JM, Lee YS. CX3CL1-Fc treatment prevents atherosclerosis in Ldlr KO mice. Mol Metab 2019;20:89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221128573 for CX3CR1 deficiency exacerbates immune-mediated hepatitis by increasing NF-κB-mediated cytokine production in macrophage and T cell by Mi Ren, Jinyan Zhang, Shen Dai, Chenxiao Wang, Zheng Chen, Siqi Zhang, Junming Xu, Xuebin Qin and Fengming Liu in Experimental Biology and Medicine