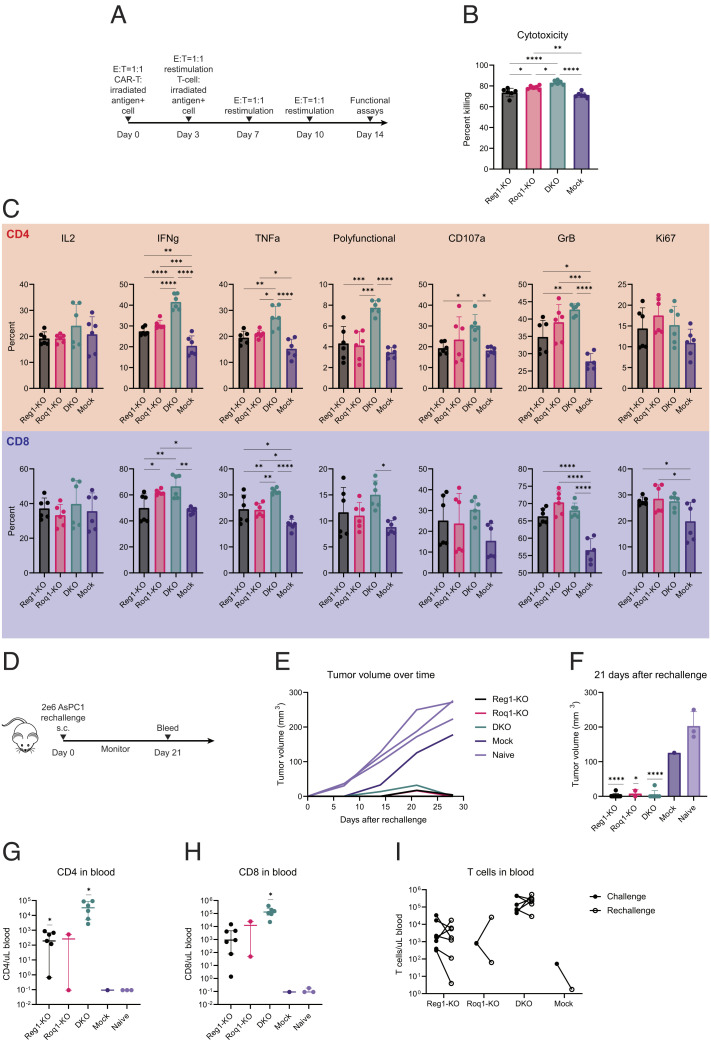
Fig. 4.
Regnase-1 and Roquin-1 double knockout enhances functional persistence. (A) Schematic of the in vitro restimulation assay to assess functional persistence. mesoCAR-T cells were cocultured with irradiated mesothelin-positive K562 cells at a 1:1 E:T ratio for a total of four stimulations. At each restimulation, T cells were counted by flow cytometry and used to seed the next restimulation at a 1:1 E:T ratio (with respect to total live T cell count, not CAR+ count) in fresh media. Cells were taken for functional assays such as cytotoxicity and intracellular cytokine staining at the fourth stimulation. (B) Cytotoxicity of serially restimulated CAR-T cells at various E:T ratios. Data shown are pooled from triplicate experiments with two independent donors. One-way ANOVA followed by Tukey’s multiple comparisons test was used for statistical analysis. (C) Cytokine expression of serially restimulated CD4 (Top) and CD8 (Bottom) T cells. T cells were treated with PMA and Ionomycin for an hour before addition of Brefeldin A and Monensin, additional 4-h culture time, and intracellular cytokine staining of Th1 cytokines (IL2, IFNg, TNFa) and degranulation-associated proteins (CD107a, GrB). Polyfunctional indicates simultaneous expression of IL2, IFNg, and TNFa. Data shown are pooled from triplicate experiments with 2 independent donors. (D) Schematic of in vivo mouse experiments to evaluate persistence of knockout CAR-T cells and response to tumor rechallenge. Mice that demonstrated a complete response (cleared tumor) from antitumor efficacy studies were used for these experiments. Tumor-free mice were rechallenged with injection of 2 × 106 AsPC1 cells subcutaneously on the opposite (Left) flank of the original tumor at least a month after initial tumor clearance. The number of mice per group could not be strictly controlled due to differential responses to initial tumor challenge. Naïve mice that had not been previously challenged with tumor cells were used as controls for tumor growth. Mice were monitored, weighed, and measured with calipers once a week and bled after 21 d. (E and F) Reponses to tumor rechallenge by knockout and mock CAR-T cells following at least a month from primary tumor clearance. Mice for this experiment (n = 8 Reg1-KO; n = 2 Roq1-KO; n = 7 DKO; n = 1 Mock; n = 3 Naive) were taken from two independent experiments. Power analysis was not used to determine sizes of each group. (E) Tumor growth over time after rechallenging previously treated mice with AsPC1 cells. (F) Tumor sizes 21 d after rechallenge. One-sample t tests were used for pairwise statistical analysis between knockout groups versus mock since the experiment only contained one mock-treated mouse. Error bars represent SD. (G and H) Quantification of CD4 (Left) and CD8 (Right) T cells in peripheral blood 21 d following tumor rechallenge. Some data points are not shown due to 0 values on a log-scale plot. Horizontal lines represent median. One-sample t tests were used for pairwise statistical analysis between knockout groups versus mock. (I) Changes in peripheral T cell numbers between 21 d after initial tumor challenge and 21 d after tumor rechallenge in rechallenged tumor-cleared mice. The number of mice per group could not be strictly controlled due to differential responses to initial tumor challenge. One-way ANOVA followed by Dunnett’s multiple comparisons test was used for statistical analysis. Not shown = not significant, *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.