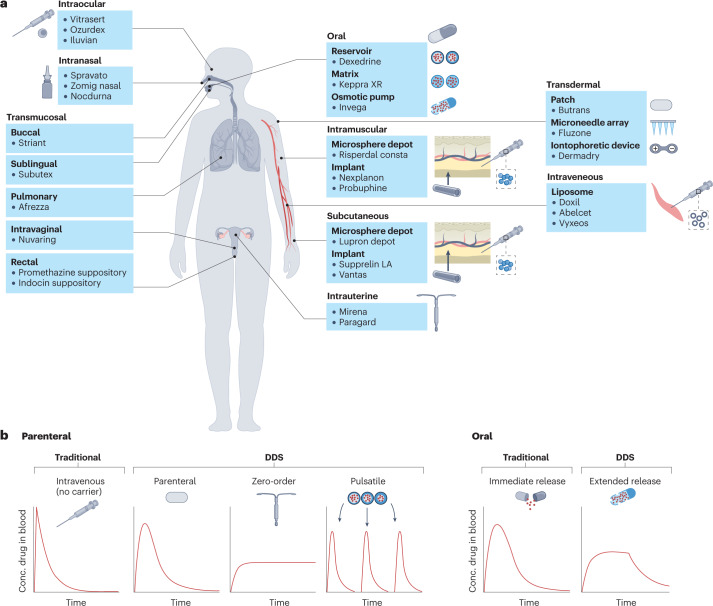
Fig. 2. Drug delivery system examples and characteristics.
a, US Food and Drug Administration (FDA)-approved examples of drug delivery systems (DDSs) grouped by route of administration (oral, intramuscular, transdermal, subcutaneous, intraocular, intranasal, intrauterine and transmucosal (pulmonary, sublingual, buccal, intravaginal and rectal)). b, Pharmacokinetic profiles showing the plasma concentration of a drug following a single dose, based on the type of release. Traditional, non-DDS formulations of parenteral and oral drugs result in rapid clearance of drug from the blood, whereas some DDSs can prolong the duration over which the drug concentration remains within the therapeutic window without increasing the peak drug concentration. In the case of pulsatile release, DDSs can also allow for multiple, pre-programmed release events mimicking bolus doses of drug following a single administration.