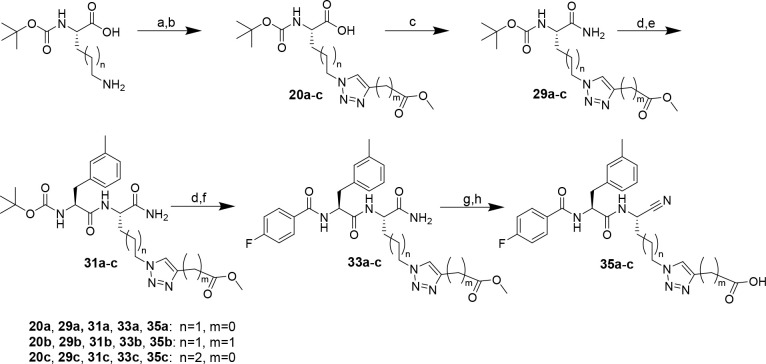
Scheme 4. Synthesis of Dipeptide Nitriles 35a–c with a Carboxy-Functionalized 1,2,3-Triazolyl Residue at P1.
Reagents and conditions: (a) triflyl azide, CuSO4·5H2O, K2CO3, MeOH/H2O (3:1), overnight; (b) methyl propiolate or methyl butynoate, CuSO4·5H2O, sodium ascorbate, DMSO/H2O (2:1), overnight; (c) iBCF, NMM, NH3, THF, −15 °C, 10 min, rt, 30 min; (d) TFA/CH2Cl2 (1:1), 2 h; (e) Boc-3-methyl-l-phenylalanine, DIPEA, PyBOP, THF, 3 h; (f) 4-fluorobenzoyl chloride, TEA, CH2Cl2, 2 h; (g) cyanuric chloride, DMF, 3 h; (h) NaOH, THF/MeOH (3:1), overnight or pig liver esterase, KH2PO4 buffer (0.2 M, pH 7.0)/acetone (10:1), 6–10 days.