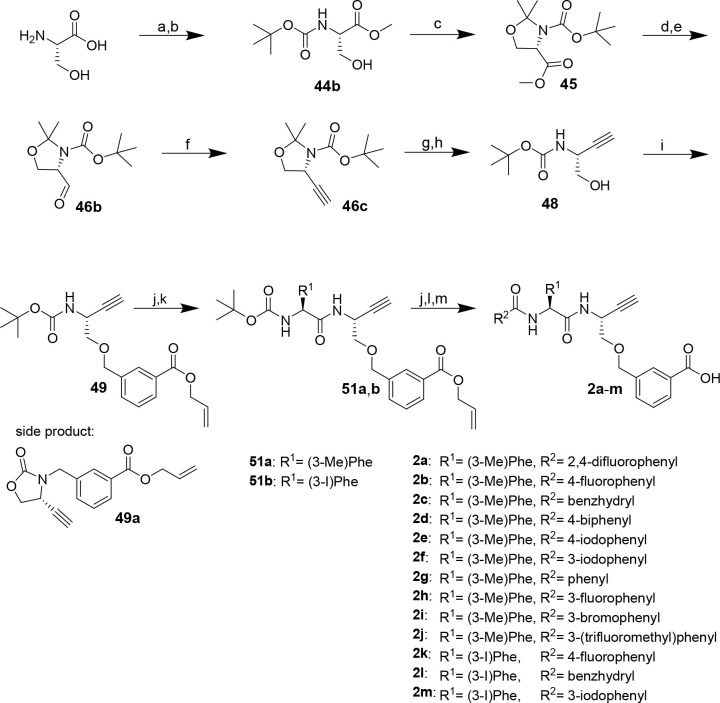
Scheme 6. Synthesis of Dipeptide Alkynes 2a–m.
Reagents and conditions: (a) acetyl chloride, MeOH, reflux, 2 h; (b) Boc2O, TEA, THF, 0 °C, 45 min, rt, overnight; (c) 2,2-dimethoxypropane, BF3·OEt2, acetone, 3 h; (d) LiAlH4, THF, 45 min; (e) oxalyl chloride, DIPEA, DMSO, CH2Cl2, −78 °C, 80 min, 0 °C, 10 min; (f) dimethyl (1-diazo-2-oxopropyl)phosphonate, K2CO3, MeOH, 0 °C, 4 h; (g) HCl (4 M)/MeOH (3:5), reflux, 1 h; (h) Boc2O, TEA, THF, 0 °C, 45 min, rt, overnight; (i) NaH, DMF, 0 °C, 5 min, rt, 1.5 h; (j) TFA/CH2Cl2 (1:1), 2 h; (k) N-Boc-amino acid, DIPEA, PyBOP, THF, 3 h; (l) acyl chloride, TEA, CH2Cl2, 2 h, or carboxylic acid, DIPEA, PyBOP, THF, 3 h; (m) Pd(PPh3)4, morpholine, CH2Cl2, 30 min.