Table 3. Kinetic Characterization of Dipeptide Alkynes with Variation at P3a.
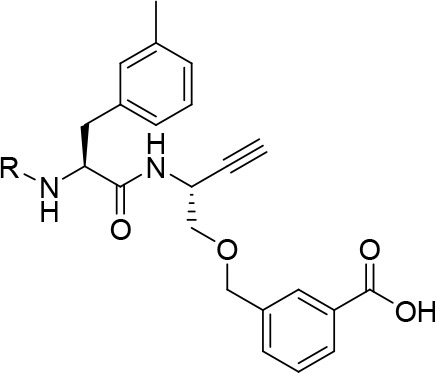
|
kinact/KI (M–1 s–1) |
|||||
|---|---|---|---|---|---|
| compound | R | CatB | CatS | CatL | CatK |
| 2a | 2,4-difluorobenzoyl | 22(2) | 58(1) | 30(3) | 3(0.1) |
| 2b | 4-fluorobenzoyl | 85(3) | 682(85) | 281(30) | 48(5) |
| 2c | diphenylacetyl | 771(17) | 47(11) | 381(43) | n.i. |
| 2d | 4-phenylbenzoyl | 41(1) | n.i. | n.i. | n.i. |
| 2e | 4-iodobenzoyl | 152(2) | 113(10) | 82(6) | 476(65) |
| 2f | 3-iodobenzoyl | 45(1) | n.i. | 1968(153) | n.i. |
| 2g | benzoyl | 88(8) | 654(48) | 222(6) | 33(6) |
| 2h | 3-fluorobenzoyl | 109(5) | 1579(114) | 483(54) | 27(2) |
| 2i | 3-bromobenzoyl | 87(29) | 570(47) | 1309(12) | n.i. |
| 2j | 3-trifluoromethylbenzoyl | 29(1) | 141(18) | 327(17) | n.i. |
The measurement was performed in three independent experiments (each as a duplicate determination) in assay buffer (pH 6.0) containing 1.5% DMSO. n.i. = no inhibition; i.e., no evidence of irreversible inhibition was discernible within the considered time and concentration ranges. Data shown are mean values ± SEM of three experiments, each performed in duplicate.
