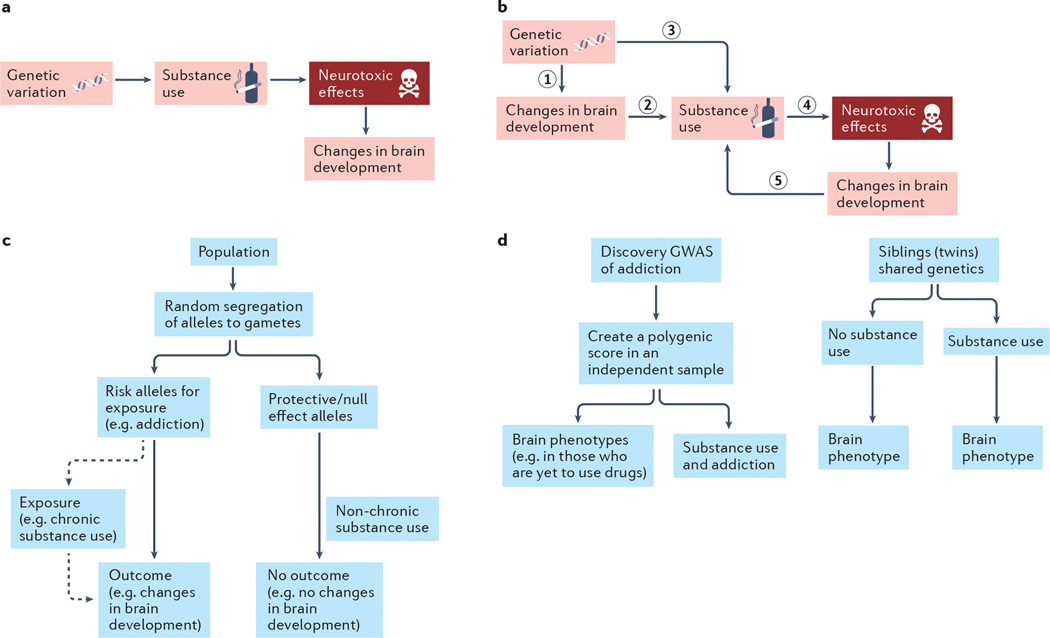
Fig. 3: Using genomics to validate hypotheses of addiction.
a| According to neurobiological models of addiction, genetic variation influences substance use, which may, in turn, exert neurotoxic effects that alter brain development. b| According to predispositional models of addiction, genetic risk for substance use disorders impacts brain development (1) prior to or concurrent with the onset of and escalating substance use and sets the neurobiological stage for substance use and future addiction (2). Consequent substance involvement (3) (also influenced by genetic risk that is not associated with neural phenotypes) may then causally influence the brain, via neurotoxic mechanisms, to further potentiate problematic substance use (4). Cyclically, these brain-related changes may further enhance risk for addiction progression (5). c| Mendelian randomization115 and other genetic causal methods can be used to evaluate these models. These approaches are based on the fact that parental genotypes conferring risk of exposure (i.e., chronic substance use) are equally as likely to be inherited by the offspring as genotypes that are protective or of no effect. Individuals inheriting risk alleles or polygenic risk of substance use will subsequently be more likely to use drugs; we can then test whether this chronic use causally alters brain development. In this method, the individual risk alleles or the polygenic risk of drug exposure is the genetic instrument and an independent association between this genetic instrument and the outcome (changes in brain development), as shown in the flow chart, is possible evidence for causal effects of substance exposure on the brain. The genetic instrument is assumed to influence the outcome (changes in brain development) solely via its influence on chronic substance use (dashed line). For a greater discussion of Mendelian Randomization approaches as well as their limitations see115. d| Testing the association between polygenic risk140 for addiction and brain imaging phenotypes, including trajectories, in drug-naïve individuals (left flow chart) is an ideal approach to assess whether pre-existing brain-related differences precede addiction. Here, the effects of genetic variants are taken from a discovery GWAS of addiction and applied to a sample, ideally of individuals without a history of substance use (e.g., children), which has brain data. A polygenic score is created in this new independent sample. It is expected that this polygenic score will eventually be associated with substance use and addiction in this sample. However, if it is also associated with brain phenotypes prior to use of substances, then we can infer that genetic risk that precedes onset of substance use contributes to brain development (part b, step 1) and later substance use (part b, step.3), rather than a causal effect of substance use on the brain alone (part b, step.4). Alternatively, examining twins (or similarly aged non-twin siblings) that are discordant117 for substance involvement can provide information on whether substance-related neural phenotypes arise from predispositional influences and/or are induced through substance involvement (right flow chart). If the brains of genetically similar individuals differ as a function of their substance use, then non-genetic mechanisms, including substance-induced changes, might be implicated. However, if they brain phenotypes are similar among those discordant for substance use, this would suggest that predispositional effects including shared genetic variation and environmental exposures are responsible for their associations with substance involvement.