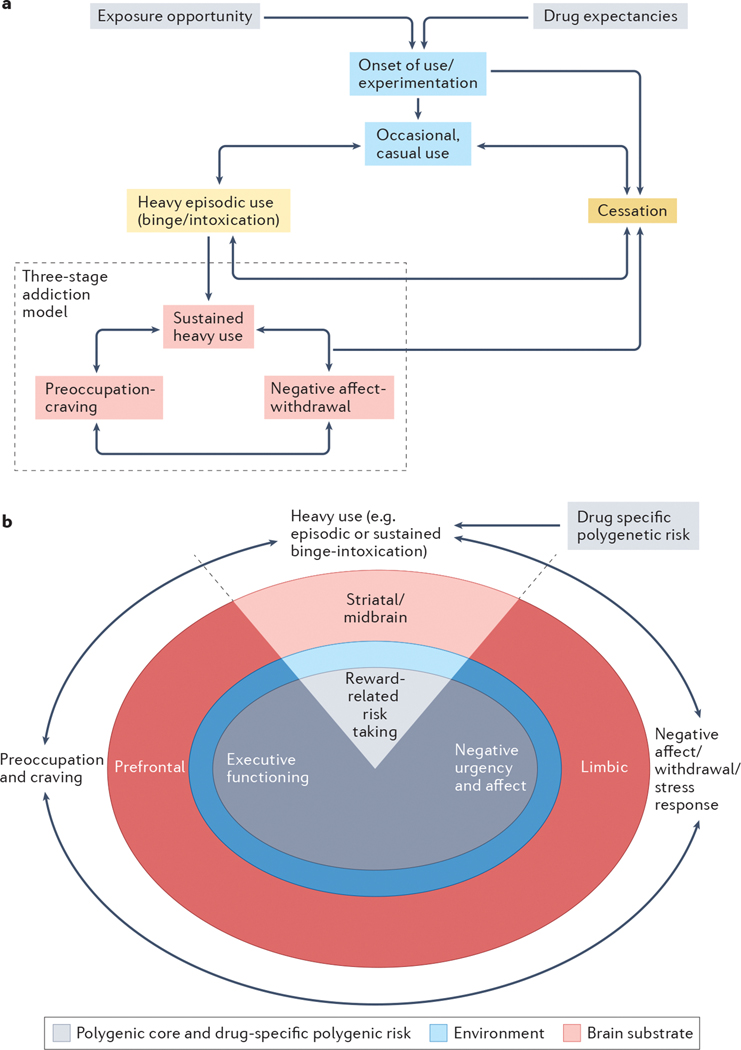
Fig. 4: A genetically informed neurobiology of addiction (GINA) model.
Addiction may be conceptualized as a developmental process or as a syndrome comprised of stages of escalating problem use. While the GINA model described here outlines a testable gene–brain–behavior mechanism underpinning the stages of addiction, it is scalable and can be extended to advance our understanding of the process of addiction. a| An illustration of the process of addiction, and those that lead into addiction, serves as a framework for understanding the GINA model. Exposure opportunity, availability193,194 and initial expectations surrounding the anticipated subjective effects of substance use serve as early contributors to drug-seeking behaviors and increase the likelihood of substance use195–198. Onset of substance use occurs in a subset of individuals, with some further entering a phase of casual but repeated substance use. Depending on the addictive potential of the substance, progression through periods of heavy episodic use and cessation may then occur (intervening aspects of these processes are not depicted). For some substances, periods of primarily reward-related occasional or casual use, heavy episodic use and cessation may occur (e.g., heavy drinking limited to college), during which time individuals may even meet criteria for milder forms of SUD199–201. Not shown are the numerous genetic and environmental influences that promote or deter progression through these substance-interfacing behaviors. For a further subset of individuals, heavy episodic use advances into a phase of sustained heavy use, wherein the pleasurable aspects of substance use are attenuated and compulsive use emerges to ameliorate negative affect, psychological and/or physiological stress states, and physiological withdrawal symptoms164. Withdrawal, and related negative mood, following substance abstinence leads to potentiated interoceptive salience through which physiological arousal associated with withdrawal and negative emotionality are potentiated21. We propose that this phase reflects moderate to severe forms of SUDs. b| The neurobiological model of addiction in the GINA framework. The GINA model places the 3 stages of addiction (shown around the outside of the image) within the context of a polygenic core (grey), environmental filter (blue) and brain substrate (pink; width of circles does not correspond to any relative magnitude of effect, i.e., genes and environment may be equally important). Each stage aligns most closely with genetic predispositions that act via specific posited brain mechanisms (these are shown “stacked” below that stage of the addiction cycle). All of the addiction stages are influenced by a polygenic core, which broadly corresponds to trait representations of substance-induced stages of sustained heavy use (binge-intoxication), negative affect (withdrawal-negative affect), and preoccupation/craving (preoccupation-anticipation) and by additional drug-specific polygenic risk that influences addiction, partly via the brain (for example, variants in genes encoding neurotransmitters) as well as via non-brain mechanisms (such as metabolic variants). Polygenic liability to reward-related risk-taking contributes to initial phases of binge-intoxication and may promote later escalating use (shown as heavy - episodic and sustained - use), which plays a role in promoting the reward related neural response to pleasurable aspects of substance use (e.g., striatal brain regions6). On the other hand, chronic substance use induces brain-related alterations that culminate in heightened stress states and negative affect (for instance, those with polygenic liability to negative urgency may be more vulnerable to this pathway) via a modified limbic response6. Furthermore, polygenic liability to executive function is likely to be instrumental in drug craving via changes in prefrontal brain function that results in increasing difficulties regulating the emotional salience of substance-related stimuli, despite the potential of subjective and cognitive desires to stop. Despite the appearance in this schematic of a one-to-one correspondence between polygenic liability, brain region and addiction stage, the gene–brain–behavior map is likely to be more interconnected. For instance, sustained heavy use in the context of negative affect may be influenced by polygenic risk to negative urgency and affect, via limbic pathways as well as substance-induced alterations in striatal circuits. The environment provides a filter for genetic liability (i.e., the magnitude and nature of genetic effects may be different in differing environmental contexts) and also directly underpins addictions. The brain is depicted as the outer substrate from which psychological aspects of addiction emerge. While distinct brain systems are illustrated, it is likely that networks of brain regions correspond to the three stages of addiction. While not noted here, aspects of addiction arise from and impact other bodily systems as well as the brain.