Abstract
Background:
Cascade genetic testing for hereditary cancer syndromes offers affected relatives the opportunity to pursue cancer screening and risk-reducing surgery, reducing morbidity and mortality. The purpose of this study was to measure long-term utilization of targeted cancer prevention and quality of life among at-risk relatives offered clinician-facilitated cascade genetic testing.
Methods:
In a pilot study, at-risk relatives of patients with a hereditary cancer syndrome were contacted directly by the clinical team and offered telephone genetic counseling and genetic testing via an at-home, mailed saliva kit. Here, we report the two-year follow-up results evaluating use of targeted cancer prevention strategies and quality of life for enrolled relatives. Quality of life was measured with validated surveys, and scores were compared to time of initial contact by the Wilcoxon signed-rank test.
Results:
Ninety-five at-risk relatives were enrolled in the initial pilot study and 72 (76%) participated in two-year follow-up, among which 57 (79%) had completed genetic testing. Of those 57 relatives, 25 were found to harbor an inherited pathogenic variant. Eighteen relatives were recommended guideline-based cancer surveillance; among whom 13 (72%) completed at least one recommended screening and 6 (33%) completed all recommended screenings. Ten relatives were recommended risk-reducing surgery; among whom 4 (40%) completed a total of 8 procedures. Quality of life surveys demonstrated low levels of anxiety, depression, distress, and uncertainty.
Conclusions:
Our two-year follow-up of the original pilot study revealed that clinician-facilitated cascade testing resulted in genetically targeted cancer screening and prevention with preserved quality of life. These results suggest that medical systems should consider supporting clinician-facilitated cascade testing programs, to be confirmed by larger randomized controlled trials.
Keywords: Cancer screening, Genetic counseling, Prevention, Cascade testing, Genetic testing, Cancer prevention, Hereditary cancer syndromes, Early detection
Precis:
Cascade testing for cancer syndromes facilitated by the medical team resulted in identification of relatives with pathogenic variants and use of genetically targeted cancer prevention with preserved quality of life.
Introduction
Genetic testing for hereditary cancer syndromes can identify individuals carrying a pathogenic variant (mutation) before they develop cancer, maximizing disease prevention and early detection.1 The process of starting with the affected relative (proband) and extending genetic testing to at-risk relatives is termed “cascade testing.” Relatives found to carry the familial pathogenic variant can benefit from genetically-targeted primary disease prevention through intensive surveillance and risk-reducing surgery.2–5 For example, among individuals with BRCA1/2 pathogenic variants, risk-reducing bilateral salpingo-oophorectomy and bilateral mastectomy are associated with a decreased risk of breast cancer, and salpingo-oophorectomy is associated with a decreased risk of ovarian cancer as well as a significantly lower all-cause mortality rate.6 Among individuals with Lynch syndrome, colonoscopic screening decreases the risk for colorectal cancer, prevents colorectal cancer deaths, and decreases overall mortality.7, 8
The Centers for Disease Control and Prevention Office of Public Health Genomics has designated cascade genetic testing as a tier one genomic application for hereditary breast and ovarian cancer as well as Lynch syndrome.,9 However, the literature suggests that only about a third of at-risk relatives undergo recommended genetic testing. Low rates of cascade genetic testing are likely due in part to the current paradigm whereby the patients/probands shoulder the burden for coordinating relative testing.10–14 In a previous pilot study, we evaluated the feasibility of clinician-facilitated cancer cascade testing with direct relative contact by the clinical team, telephone genetic counseling, and mailed saliva kit testing.15 The clinician-facilitated strategy yielded promising results, achieving cancer genetic testing for 58% of total identified relatives, and for 70% of relatives who were able to be reached by the research team.15 These results have been confirmed by a systematic review and meta-analysis, demonstrating cascade testing completion for 36% of relatives with patient-mediated relative contact versus 63% of relatives when relatives are directly contacted by the medical team or testing laboratory.16 Although this increased utilization of genetic testing is exciting, it is only clinically meaningful if action is taken in response to positive genetic testing results. To promote a paradigm shift, whereby clinicians and medical centers embrace the responsibility of cascade testing, data on long-term health and quality of life implications for at-risk relatives are needed. The aim of this study was to evaluate the two-year follow-up of a novel method of enhanced cascade testing, focusing on utilization of cancer screening, cancer risk-reducing surgery, and quality of life following the receipt of positive genetic results.
Methods
Study participants (probands and relatives)
Patient (proband) eligibility for facilitated cascade testing was screened prior to enrollment in the pilot study. Inclusion criteria required that patients were at least 18 years old, diagnosed with an autosomal dominant hereditary cancer syndrome within the preceding 12 months, and had at least one at-risk relative. Probands were treated at a single academic institution where genetic testing occurred following in-person genetic counseling. At-risk relatives were defined as blood relatives of the proband who are at risk for carrying the pathogenic variant present in the proband. The first step in the facilitated cascade testing pathway involved a meeting between the proband and the genetics team to review the family pedigree and specific pathogenic variant. The genetics team comprehensively reviewed the family history and identified the relatives at-risk for the pathogenic variant. The study team encouraged probands to offer participation to all first-degree relatives, however, if there were additional non-first-degree relatives at-risk and interested in testing, they also could be offered participation (e.g., first cousins where the presumed shared relative had passed away and therefore could not have confirmatory genetic testing). This process was the same for all identified pathogenic variants. Relatives were eligible for inclusion if the proband granted the study team permission to contact them, they were 18 years of age or older, lived in the United States, and had not undergone prior genetic testing for cancer-associated hereditary pathogenic variants. Probands were advised to contact their designated relatives prior to contact by the genetics team. Probands were then re-contacted prior to relative contact by the genetics team to confirm that the information had been shared with relatives. All probands and relatives provided written informed consent for study participation. Additional protocol details are available in the original pilot publication.15
Thirty probands and 95 relatives were enrolled in the previously published cascade testing feasibility trial.15 Probands initially identified 114 at-risk relatives; however, 19 were excluded for the following reasons: relative lived outside of the United States (5), proband decided that she/he did not feel comfortable sharing the genetic information with relatives (7), and relative did not respond to three attempts at telephone contact by the study team (7). Among the original 114 identified relatives, 66 (58%) completed genetic testing. Among the remaining 95 relatives successfully contacted by the study team, 66 (70%) completed genetic testing. In the present study, the 95 enrolled relatives were called by telephone for participation in the two-year follow-up.
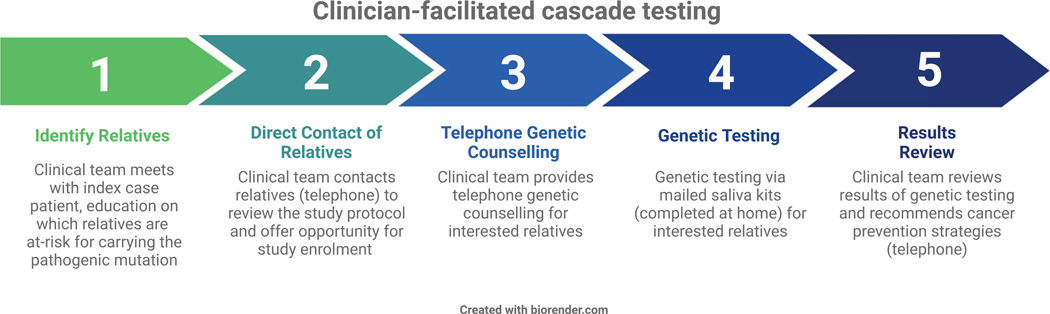
Trial design
This prospective study was approved by the Weill Cornell Medicine Institutional Review Board. The facilitated cascade testing pathway, including a bundle of five separate components, has previously been published (Figure 1).15 Current standard of care relies on the affected proband to contact at-risk relatives, disclose the familial pathogenic variant, and encourage cascade testing. With our pilot study’s intervention, the clinical team directly contacted relatives for results disclosure and offered to facilitate the genetic testing process through telephone genetic counseling and mailed saliva kits for at-home sample collection. The cost of genetic testing for at-risk relatives was covered as part of study participation. A single member of the clinical team performed all telephone calls and utilized a script for the discussion to allow for consistency in the intervention. Relatives that completed genetic testing were contacted by the same study team member and a certified genetic counselor (provided by a third-party genetic counseling service with licensure across several states) for post-test genetic counseling, which included results disclosure and, for those with the pathogenic variant, a review of National Comprehensive Cancer Network (NCCN)-based cancer prevention and early detection guidelines.17, 18
Figure 1.
Clinician-facilitated cascade testing
Relatives were contacted again by telephone for follow-ups at six months and at two years. As part of the follow-up protocol, relatives were emailed quality of life surveys to complete. Relatives found to have a cancer-associated pathogenic variant were asked by telephone, using a script, whether or not they had completed cancer screening interventions or surgeries that were recommended based on the NCCN guidelines as a direct result of their cascade testing results. If a relative had already completed or planned a cancer screening or surgery independent of the genetic testing results, this was not included, as the goal of this portion of the study was to measure direct downstream implications of cascade testing. As the NCCN guidelines are frequently updated and include both evidence-based recommendations and considerations when the data are less clear, we only assessed for uptake of cancer prevention strategies that were recommended and did not change over the course of the study period.
Endpoints
The primary outcomes were rate of utilization of recommended cancer surveillance and risk-reducing surgery and relative-reported quality of life at two-year follow-up. The recommendations for cancer surveillance and risk-reducing procedures were gene-, age-, and biologic sex-specific and based on the NCCN guidelines.17, 18 The validated quality of life survey instruments included the Hospital Anxiety and Depression Scale (HADS),19 Satisfaction with Decision Scale (SDS),20 and the Multidimensional Impact of Cancer Risk Assessment (MICRA) Questionnaire.21 Exploratory outcomes included the evaluation of the contribution of relative demographics to completion of genetic testing, utilization of recommended cancer screening and prevention strategies, and quality of life results. All relative demographics, including race and ethnicity, were obtained by self-report.
Statistical Analysis
The distribution of continuous variables was tested for normality via the Kolmogorov–Smirnov test. Univariate tests were applied based on whether the variable of interest was distributed normally (i.e., t-test, analysis of variance) or not normally (i.e., Mann–Whitney U test, Kruskal-Wallace test, Friedman test). Associations between categorical variables were evaluated by the chi-square test or Fisher’s exact tests, as appropriate for category size. Quality of life scores on the instruments at six-month and two-year follow-up were compared to time of initial relative contact for cascade testing by the Wilcoxon signed-rank test. All P-values are two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in SPSS Version 24 (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.).
Results
Relative demographics
Among the 95 relatives enrolled in the initial pilot study, 72 (76%) agreed to participate in long-term follow-up. Of these 72 relatives, 36 (50%) identified as female and 36 (50%) as male. The median age of relatives was 50 years (range 41–66). The most common pathogenic variants present in the proband for participating relatives were located in the following genes: BRCA1 (27), BRCA2 (13), BRIP1 (10), APC (5), MSH6 (4), PTEN (4) and RAD51C (4). Relatives self-reported both race and ethnicity. Among the 72 relatives, 57 (79%) were White, 9 (13%) were Asian, and 6 (8%) were Black; 69 (96%) identified as non-Hispanic or Latino and 3 (4%) identified as Hispanic or Latino (Table 1). Thirteen relatives (18%) reported a personal history of cancer. Twelve relatives (17%) reported their highest level of education as high school, 34 (47%) as college, and 22 (31%) as graduate school. There were no significant differences in any of the relative characteristics when comparing the population from the initial pilot study and the subgroup participating in long-term follow-up.
Table 1.
Relative demographics (N=72)
| Age (median, range) | 50 years (41–66) | |
|---|---|---|
| Race | N | % |
| White | 57 | 79.2 |
| Asian | 9 | 12.5 |
| Black | 6 | 8.3 |
| Ethnicity | ||
| Not Hispanic/ Latino | 69 | 95.8 |
| Hispanic/Latino | 3 | 4.2 |
| Sex | ||
| Female | 36 | 50 |
| Male | 36 | 50 |
| Children | ||
| No | 23 | 31.9 |
| Yes | 49 | 68.1 |
| Pathogenic variant present in proband | ||
| BRCA1 | 27 | 37.5 |
| BRCA2 | 13 | 18.1 |
| BRIP1 | 10 | 13.9 |
| APC | 5 | 6.9 |
| MSH6 | 4 | 5.6 |
| PTEN | 4 | 5.6 |
| RAD51C | 4 | 5.6 |
| CHEK2 | 3 | 4.2 |
| Personal history of cancer | ||
| No | 59 | 81.9 |
| Yes | 13 | 18.1 |
Cascade Genetic Testing
In the initial feasibility trial, 66 (70%) of the 95 enrolled relatives completed genetic testing. Among the 72 relatives participating in this long-term follow-up study, 57 (79%) completed genetic testing for the familial pathogenic variant and 15 (21%) did not. Among the 57 relatives undergoing genetic testing included in the follow-up study, 27 (47%) had positive genetic testing (demonstrating the pathogenic variant identified in the proband). Two relatives were found to have MUTYH heterozygous pathogenic variants, however, these relatives were not included in long-term follow-up evaluation of cancer risk-reducing strategies as the autosomal dominant pathogenic variant did not impact management recommendations. Twenty-five relatives had genetic testing demonstrating pathogenic variants for autosomal dominant hereditary cancer syndromes. None of the relatives were found to have unexpected pathogenic variants (pathogenic variants that were not present in the proband).
Genetically Targeted Primary Disease Prevention
Twenty-five (100%) of the relatives found on cascade testing to carry the familial pathogenic variant received post-test genetic counseling individualized to the specific identified variant. Based on the affected gene, relative age, biologic sex, and personal medical history, 18 relatives were counseled that they were candidates for cancer surveillance based on NCCN guidelines. Relatives were queried about completion of the following NCCN-based cancer screening interventions: BRCA1 – mammogram and breast MRI; BRCA2 – mammogram, breast MRI, and prostate specific antigen for males; CHEK2 – mammogram, breast MRI, and colonoscopy; APC I1307K variant – colonoscopy. Thirteen (72%) relatives reported completing at least one cancer surveillance test by two-year follow-up as a direct result of the cascade testing results, including 15 total screening tests. Six (33%) relatives reported completing all recommended cancer surveillance tests by two-year follow-up and 5 (28%) reported completing none of the recommended screenings. Fourteen relatives were encouraged to complete breast self-exams and provider breast exams. Among this group, 10 (71%) reported completing breast self-exams and 8 (57%) as a direct result of the counseling (Table 2).
Table 2.
National Comprehensive Cancer Network (NCCN) guideline-based cancer surveillance completed as a direct result of cascade genetic testing (N = 25 relatives with pathogenic variants)
| Recommendation for cancer screening | N | % | |
|---|---|---|---|
| Relatives not recommended cancer screening | 7 | ||
| Relatives recommended cancer screening | 18 | ||
| Completed ≥1 screening | 13 | 72.2 | |
| Completed all recommended screening | 6 | 33.3 | |
| Completed none of the recommended screening | 5 | 27.8 | |
| Screening interventions | # Relatives recommended | # Relatives completed | % |
| Mammogram | 10 | 7 | 70.0 |
| Breast magnetic resonance imaging (MRI) | 10 | 3 | 30.0 |
| Prostate specific antigen screening | 1 | 1 | 100.0 |
| Colonoscopy | 5 | 4 | 80.0 |
Measured NCCN-based cancer screening interventions by mutation: BRCA1 – mammogram, breast MRI; BRCA2 – mammogram, breast MRI, prostate specific antigen for males; CHEK2 – mammogram, breast MRI, colonoscopy; APC I1307K variant – colonoscopy.
Among the 25 relatives with positive genetic testing, 10 relatives were counseled at the time of their genetic testing results disclosure that they were candidates for risk-reducing surgery based on NCCN guidelines. The remainder of relatives were not counseled on risk-reducing surgery due to the specific pathogenic variant, sex, age, and/or prior history of risk-reducing surgery. Among the candidates for risk-reducing surgery, four relatives (40%) reported completing a total of eight risk-reducing surgical procedures as a direct result of the cascade testing results. Risk-reducing surgical procedures included bilateral salpingo-oophorectomy (4), hysterectomy (3), and mastectomy (1). Two relatives reported that they were unable to complete the NCCN recommendations due to lack of insurance coverage for cancer preventative strategies.
Relative demographics, cascade testing and cancer prevention
Relative age, race, ethnicity, sex, personal history of cancer, parenthood, and the specific familial pathogenic variant were all evaluated to identify predictors of cascade testing completion as well as utilization of recommended cancer prevention and early detection strategies. Among 57 White relatives, 42 (74%) completed genetic testing; among 6 Black relatives, 6 (100%) completed genetic testing; among 9 Asian relatives, 9 (100%) completed genetic testing (Table 1). None of the relative characteristics were associated with completion of cascade testing, recommended cancer screening, or risk-reducing surgery.
Cascade of the cascade
Among the 25 relatives found to carry the familial cancer syndrome, 22 (88%) reported sharing information about the pathogenic variant with at least one additional relative. See Table 3 for additional information on genetic testing and targeted cancer prevention among additional relatives.
Table 3.
Downstream family implications of cascade testing for 25 relatives found to carry the familial pathogenic variant
| Downstream implications of facilitated cascade testing | N (%) |
|---|---|
| Shared information about the pathogenic variant with ≥ 1 additional relative | 22 (88%) |
| ≦ 1 additional relative completed genetic testing | 15 (60%) |
| ≥ 1 additional relative had genetic testing positive for the familial pathogenic variant | 10 (40%) |
| ≦ 1 additional relative completed cancer screening based on genetic testing | 7 (28%) |
| ≥ 1 additional relative completed cancer risk-reducing surgery based on genetic testing | 3 (12%) |
Quality of Life Measures
Among the 72 included relatives, 32 (44%) completed quality of life surveys at time of initial contact for cascade testing, 27 (38%) at six-month follow-up, and 53 (74%) at two-year follow-up. Relatives reported high levels of satisfaction with their decision to participate in cascade testing at the time of cascade testing, at the six-month follow-up, and at the two-year follow-up. Relatives reported normal levels of anxiety and depression at time of cascade testing, six-month follow-up, and two-year follow-up. The median anxiety scores were significantly lower at two-year follow up compared to time of initial relative contact for cascade testing (3.0 [IQR 1.0, 6.0] vs. 4.0 [IQR 1.0, 8.2], respectively; P=0.002). The median depression scores were significantly lower at two-year follow up compared to time of initial relative contact for cascade testing (1.0 [IQR 0.0, 3.0] vs 2.0 [IQR 0.0, 5.3], respectively; P=0.02). The multidimensional impact of cancer risk assessment suggested a positive perception of genetic testing at all time points (Table 4).
Table 4.
Quality of life for relatives at time of contact for cascade testing, six-month and two-year follow-up
| Clinician-facilitated contact for cascade testing | 6-month follow-up | 2-year follow-up | P-Value (Wilcoxon signed-rank test) | |||||
|---|---|---|---|---|---|---|---|---|
| N | Median [IQR] | N | Median [IQR] | N | Median [IQR] | (0 vs. 6-month) | (0 vs. 2-year) | |
| SDS | 32 | 30.0 [24.0, 30.0] | 25 | 30.0 [25.0, 30.0] | 52 | 28.0 [24.0, 30.0] | 0.59 | 0.45 |
| HADS-A | 28 | 4.0 [1.0, 8.2] | 27 | 2.0 [1.0, 5.0] | 53 | 3.0 [1.0, 6.0] | 0.29 | 0.002 |
| HADS-D | 28 | 2.0 [0.0, 5.3] | 27 | 1.0 [0.0, 3.0] | 53 | 1.0 [0.0, 3.0] | 0.21 | 0.02 |
| MICRA | 28 | 26.0 [19.0, 35.0] | 24 | 23.0 [12.0, 30.0] | 47 | 25.0 [16.0, 33.0] | 0.02 | 0.27 |
HADS - Hospital Anxiety and Depression Scale. Range 0–21. Normal anxiety/depression - 0–7; Borderline anxiety/depression - 8–10; Abnormal anxiety/depression - 11–21.
SDS - Satisfaction with Decision Scale (A - Anxiety, D - Depression). Range 6–30. Higher score represents greater satisfaction.
MICRA - Multidimensional Impact of Cancer Risk Assessment. Range 0–125. Higher score represents more negative perception of testing.
Relatives were encouraged to complete all survey instruments however occasionally elected to only complete some of the instruments and/or to complete only some of the questions within the instrument.
Quality of life survey scores were also evaluated in the context of relative age, race, sex, personal and family cancer history, parenthood, completion of recommended cascade genetic testing, and genetic testing results. There were no differences in quality of life or decision satisfaction between relatives with positive and negative genetic testing results at any of the studied time points. At two-year follow-up, relatives that completed genetic testing had significantly improved satisfaction with their decision about genetic testing compared to those relatives that declined genetic testing (29.0 [IQR 25.0, 30.0] vs. 23.0 [IQR 20.5, 26.0], respectively; P=0.027). Non-White relatives had a more negative perception of their cancer risk assessment (MICRA) compared to White relatives at two-years (36.0 [IQR 26.0, 48.0] vs. 24.0 [IQR 14.0, 30.0], respectively; P=0.009). There was no difference in rates of genetic testing for females compared to males (30, 84% vs. 27, 75%, respectively; P=0.563); however, female relatives reported a more negative perception of their cancer risk assessment compared to male relatives at both the time of initial contact and two-year follow-up, higher levels of depression at time of cancer risk assessment (3.00 [IQR 1.25, 5.75] vs. 0.50 [IQR 0.00, 2.00], respectively; P=0.052), and higher levels of anxiety at two-year follow-up (4.0 [IQR 2.0, 7.8] vs. 2.0 [IQR 0.0, 5.0], respectively; P=0.029). Despite these differences, however, the overall experience for both males and females was reported as positive. Older relatives reported greater satisfaction with the experience of cascade testing compared to younger relatives at two-year follow-up (29.5 [IQR 25.0, 30.0] vs. 24.5 [IQR 22.8, 29.0], respectively; P=0.007). Relatives with a personal history of cancer reported higher levels of depression at two-year follow-up (3.00 [IQR 1.25, 7.00] vs. 1.00 [IQR 0.00, 3.00], respectively; P=0.038) (Table 5).
Table 5.
Relative demographics and quality of life survey scores at time of cascade testing and two-year follow-up
| Time of initial cascade testing (Median [IQR]) | Two -year follow-up testing (Median [IQR]) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SDS | HADS-A | HADS-D | MICRA | SDS | HADS-A | HADS-D | MICRA | ||
| Age | |||||||||
| < 50 years | 30.0 (24.0, 30.0) | 5.0 (1.0, 11.0) | 3.00 (0.50, 7.00) | 31 (24, 58) | 24.5 (22.8, 29.0) | 3.0 (2.0, 7.0) | 1.00 (0.00, 3.00) | 25 (18, 34) | |
| >= 50 years | 30.0 (24.5, 30.0) | 4.0 (1.0, 6.0) | 2.00 (0.00, 3.00) | 25 (18, 31) | 29.5 (25.0, 30.0) | 3.0 (1.0, 6.0) | 1.00 (0.00, 3.00) | 25 (15, 33) | |
| P-value | >0.9 | 0.3 | 0.4 | 0.2 | 0.007 | 0.4 | 0.8 | 0.9 | |
| Race | |||||||||
| White | 30.0 (25.0, 30.0) | 4.0 (1.0, 6.0) | 2.00 (0.00, 4.00) | 25 (18, 34) | 27.0 (24.0, 30.0) | 2.5 (1.0, 6.0) | 1.00 (0.00, 3.00) | 24 (14, 30) | |
| Non-White | 30.0 (21.0, 30.0) | 4.0 (1.0, 10.0) | 3.00 (0.50, 5.50) | 31 (26, 51) | 29.0 (24.50, 29.0) | 3.0 (2.0, 7.5) | 1.00 (0.00, 2.00) | 36 (26, 48) | |
| P-value | 0.6 | 0.7 | 0.7 | 0.11 | 0.9 | 0.3 | 0.7 | 0.009 | |
| Sex | |||||||||
| Female | 30.0 (25.5, 30.0) | 7.0 (3.2, 10.0) | 3.00 (1.25, 5.75) | 32 (26, 48) | 28.5 (24.2, 30.0) | 4.0 (2.0, 7.8) | 1.50 (0.00, 4.50) | 31 (24, 40) | |
| Male | 30.0 (23.0, 30.0) | 2.0 (0.2, 4.0) | 0.50 (0.00, 2.00) | 23 (15, 30) | 27.5 (24.0, 30.0) | 2.0 (0.0, 5.0) | 1.00 (0.00, 3.00) | 18 (12, 26) | |
| P-value | 0.5 | 0.057 | 0.052 | 0.021 | 0.8 | 0.029 | 0.2 | 0.003 | |
| Proband with history of cancer | |||||||||
| No | 30.0 (27.5, 30.0) | 2.0 (0.0, 4.0) | 1.00 (0.00, 6.00) | 26 (18, 30) | 27.0 (24.5, 30.0) | 2.0 (1.0, 5.0) | 1.00 (0.00, 3.25) | 24 (12, 31) | |
| Yes | 30.0 (24.0, 30.0) | 4.0 (1.5, 9.5) | 2.00 (0.50, 4.50) | 27 (23, 39) | 29.0 (24.0, 30.0) | 4.0 (2.0, 7.0) | 1.00 (0.00, 3.00) | 25 (18, 36) | |
| P-value | 0.4 | 0.1 | 0.6 | 0.6 | >0.9 | 0.2 | 0.8 | 0.3 | |
| Relative with history of cancer history | |||||||||
| No | 30.0 (24.0, 30.0) | 3.5 (0.8, 5.2) | 1.50 (0.00, 4.50) | 25 (18, 34) | 27.5 (24.0, 30.0) | 2.0 (1.0, 6.0) | 1.00 (0.00, 3.00) | 25 (19, 33) | |
| Yes | 30.0 (25.2, 30.0) | 4.0 (3.5, 7.0) | 2.00 (0.75, 5.50) | 27 (26, 36) | 29.0 (25.0, 30.0) | 4.0 (2.2, 7.5) | 3.00 (1.25, 7.00) | 23 (10, 43) | |
| P-value | 0.8 | 0.6 | 0.7 | 0.6 | 0.3 | 0.4 | 0.038 | >0.9 | |
| Parenthood/children | |||||||||
| No | 29.0 (21.8, 30.0) | 4.0 (4.0, 10.0) | 1.00 (1.00, 5.00) | 26 (12, 42) | 27.0 (24.2, 29.8) | 4.0 (2.5, 7.0) | 1.00 (0.00, 2.00) | 20 (9, 31) | |
| Yes | 30.0 (24.8, 30.0) | 3.0 (0.0, 8.0) | 2.00 (0.00, 5.00) | 25 (19, 34) | 28.0 (24.0, 30.0) | 2.0 (1.0, 6.0) | 1.00 (0.00, 3.75) | 26 (19, 36) | |
| P-value | 0.5 | 0.2 | 0.7 | 0.8 | >0.9 | 0.2 | 0.5 | 0.089 | |
| Genetic testing completed | |||||||||
| No | 18.0 (18.0, 18.0) | NA | NA | NA | 23.0 (20.5, 26.0) | 3.0 (2.0, 9.8) | 2.50 (1.00, 4.75) | 36 (21, 50) | |
| Yes | 30.0 (24.5, 30.0) | 4.0 (1.0, 8.2) | 2.00 (0.00, 5.25) | 26 (19, 35) | 29.0 (25.0, 30.0) | 3.0 (1.0, 6.0) | 1.00 (0.00, 3.00) | 25 (17, 33) | |
| P -value | 0.1 | 0.027 | 0.3 | 0.12 | 0.9 | ||||
HADS - Hospital Anxiety and Depression Scale, SDS - Satisfaction with Decision Scale, MICRA - Multidimensional Impact of Cancer Risk Assessment
Discussion
In this follow-up study, we have demonstrated that cascade testing offers the ability to extend genetic testing and genetically targeted disease prevention to disease-free relatives, maximizing the potential benefits of cancer genetics. Among relatives carrying a familial cancer-associated pathogenic variant, 72% completed at least one of the evidence-based recommended cancer screenings as a direct result of the cascade testing, including 15 cancer surveillance tests. Among relatives offered cancer risk-reducing surgery, 40% completed a surgery, including both breast and gynecologic risk-reducing procedures. However, only 33% of relatives completed all of the NCCN-recommended cancer screenings. To date, much of the research and clinical work in the field of cancer genetics has focused on increasing access to and utilization of genetic testing. As the true power of genetic testing relies on translation of a positive testing result into cancer prevention and early detection, our results suggest that genetic navigation programs might consider focusing on both facilitating cascade genetic testing and encouraging affected individuals to engage with the medical system. Moving forward, efforts to improve patient and community engagement among diverse patient populations through collaborations with community-based organizations, advocacy groups, and navigation programs should be considered as they have been shown to improve patient care and satisfaction in oncology and to lower costs associated with cancer care.22–24
Since the introduction of genetic sequencing technology, there has been concern that testing could provoke psychological distress. However, among individuals with a cancer diagnosis, the research to date suggests overall a positive psychological impact of genetic testing.25–29 Genetic testing for relatives may present unique complexities, as many family members will carry the mutated gene but will not develop cancer.30 Adding to the complexity, psychological models of decision reassurance suggest two components: short-term cognitive reassurance and long-term cognitive benefits, such as reduced anxiety.31 We have assessed the long-term quality of life and the psychosocial effects of clinician-facilitated cascade testing on relatives and confirmed low levels of anxiety and depression and high levels of satisfaction with the experience of cascade testing at the two-year follow-up. While prior work has suggested higher distress among individuals found to have a pathogenic variant versus those with negative results, our study found no statistically significant difference, although the study was limited by a small sample size.32 The finding of overall high levels of satisfaction with genetic testing and an improved experience among older individuals is consistent with the prior literature on cancer genetic assessment.32–35 Interestingly, while several prior studies demonstrate that females are more likely to complete cascade testing compared to males, we found no difference in rates of testing by sex in our cohort;11, 36–39 however, females reported a more negative perception of cancer risk assessment at both the time of initial contact and the two-year follow-up, as compared to males. Larger, adequately powered studies are needed to evaluate the contribution of age, race, ethnicity, sex, insurance status, and social determinants of health to the psychosocial experience with cascade genetic testing.
This study has several limitations. Initial relative contact and enrollment was performed by a single clinician without review by other team members to check for fidelity. Future studies should include mechanisms to review this contact and enrollment process in order to develop validated, reproducible, and scalable approaches to relative communication. The study was only able to reach 76% of enrolled relatives for long-term follow-up. For relatives that avoided contact, we do not have information on completion of genetic testing, utilization of cancer screening and prevention, or quality of life, so our reported utilization of medical interventions and relative satisfaction may be overstated. Similarly, we cannot assess any perceptions of intrusion or violation of privacy, as those relatives feeling this way did not provide follow-up data. Prior literature suggests racial and ethnic disparities in genetic medicine.40–45 While our results did not demonstrate disparities, this was a small feasibility trial without a control arm and with limited racial and ethnic diversity. Therefore, we could not make any definitive conclusions on race, ethnicity, or education level as they relate to cascade genetic testing. A follow-up multi-institutional randomized controlled study of clinician-facilitated cascade testing for BRCA1/2 pathogenic variants is ongoing to address some of these important limitations.46 This study included probands with several autosomal dominant hereditary cancer syndromes with varying levels of penetrance and clinical actionability, including APC 1307K and CHEK2, where the absolute cancer risk is low and the role for cancer screening remains uncertain. Furthermore, we did not limit enrollment to first-degree relatives and therefore relatives had varying levels of risk for carrying the familial pathogenic variant based on degree of relation to the proband. This heterogeneity may have contributed to differences in cascade testing uptake and utilization of cancer preventative and screening interventions. Utilization of NCCN-recommended cancer screening interventions and risk-reducing surgery was based on relative self-report. Future studies may consider confirming utilization of such interventions by review of medical records.
Cost is a critical driver in patient compliance to receive genetic testing and therefore must be addressed when considering implementing cascade testing programs.47 This study provided free genetic testing and counseling and, as a research initiative, avoided some of the complexities in state-specific genetic counseling licensure regulations (probands and relatives provided informed consent on an institutional review board-approved clinical trial and a third-party genetic counseling service with licensure across several states was used). Despite a growing body of literature suggesting the cost-effectiveness of genetic testing and cascade testing programs, additional well-designed prospective trials of facilitated cascade testing are crucial before medical systems will invest in the necessary resources and infrastructure.48–50 While many genetic testing laboratories have developed programs to offer free testing for relatives of an affected individual and have employed tele-genetics teams with licensure that extends across the United States, sustainable cascade testing programs will require medical systems to commit financial support and resources. In order to demonstrate sustainability, future research is needed to evaluate the feasibility and cost-effectiveness associated with genetic counseling, sequencing, and cancer preventative services per additional quality-adjusted life year.
In summary, our long-term follow-up study demonstrates that clinician-facilitated cascade testing can result in successful completion of genetic testing and utilization of genetically targeted primary disease prevention through screening and risk-reducing surgery with a positive patient-reported experience. These results, supported by a recent meta-analysis,16 suggest that healthcare systems should consider supporting clinician-facilitated direct contact cascade testing programs, to be confirmed by larger randomized controlled trials. Notably, 67% of relatives did not complete all of the NCCN-recommended cancer screening interventions, suggesting that future strategies to promote care for families with hereditary cancer syndromes must focus not just on access to genetic testing but also on supporting engagement with the medical system following identification of a germline pathogenic variant.
Acknowledgement of research support:
Melissa K. Frey is supported by the following grant: NIH/NCATS Grant # KL2-TR-002385. Ravi N. Sharaf was supported by the following grants: National Cancer Institute Grant # K07CA216326 and R01CA211723 and Relative Centered Outcomes Research Institute Grant # IHS-2017C3-9211.
Paul J. Christos and Charlene Thomas were supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
Steven Lipkin was supported by the following grant: National Cancer Institute Grant #U01CA233056.
Roni Nitecki is supported by the following grant: NIH T32 (5T32 CA101642)
J. Alejandro Rauh-Hain is supported by the following grant: NIH (K08CA234333)
We acknowledge Dr. Alvin Mushlin for his generous contribution to the quality of life assessments in this manuscript.
Kevin Holcomb serves as a consultant for Johnson and Johnson and receives research support from Fujirebio Diagnostics, outside the submitted work.
Footnotes
Conflict of Interest Disclosures:
The other authors made no disclosures.
Contributor Information
Melissa K. Frey, Weill Cornell Medicine, New York, NY.
Muhammad Danyal Ahsan, Weill Cornell Medicine, New York, NY.
Nora Badiner, Weill Cornell Medicine, New York, NY.
Jenny Lin, Weill Cornell Medicine, New York, NY.
Priyanka Narayan, Weill Cornell Medicine, New York, NY.
Roni Nitecki, Anderson Cancer Center, Houston, TX.
Jose Alejandro Rauh-Hain, Anderson Cancer Center, Houston, TX.
Haley Moss, Duke University Medical Center, Durham, NC.
Rana Khan Fowlkes, Weill Cornell Medicine, New York, NY.
Charlene Thomas, Weill Cornell Medicine, New York, NY.
Hannah Bergeron, Weill Cornell Medicine, New York, NY.
Paul Christos, Weill Cornell Medicine, New York, NY.
Sarah R. Levi, Weill Cornell Medicine, New York, NY.
Stephanie V Blank, Icahn School of Medicine, New York, NY.
Kevin Holcomb, Weill Cornell Medicine, New York, NY.
Evelyn Cantillo, Weill Cornell Medicine, New York, NY.
Ravi N. Sharaf, Weill Cornell Medicine, New York, NY.
Steven Lipkin, Weill Cornell Medicine, New York, NY.
Kenneth Offit, Memorial Sloan Kettering Cancer Center, New York, NY.
Eloise Chapman-Davis, Weill Cornell Medicine, New York, NY.
References
- 1.Childers CP, Childers KK, Maggard-Gibbons M, Macinko J. National Estimates of Genetic Testing in Women With a History of Breast or Ovarian Cancer. J Clin Oncol. Dec 2017;35(34):3800–3806. doi: 10.1200/JCO.2017.73.6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Practice CoG. ACOG Committee Opinion No. 727: Cascade Testing: Testing Women for Known Hereditary Genetic Mutations Associated With Cancer. Obstet Gynecol. January 2018;131(1):e31–e34. doi: 10.1097/AOG.0000000000002457 [DOI] [PubMed] [Google Scholar]
- 3.Randall LM, Pothuri B, Swisher EM, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol. August 2017;146(2):217–224. doi: 10.1016/j.ygyno.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Offit K. The future of clinical cancer genomics. Semin Oncol. Oct 2016;43(5):615–622. doi: 10.1053/j.seminoncol.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. May 2014;32(15):1547–53. doi: 10.1200/JCO.2013.53.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, You R, Wang X, et al. Effectiveness of Prophylactic Surgeries in BRCA1 or BRCA2 Mutation Carriers: A Meta-analysis and Systematic Review. Clin Cancer Res. August 2016;22(15):3971–81. doi: 10.1158/1078-0432.CCR-15-1465 [DOI] [PubMed] [Google Scholar]
- 7.Engel C, Rahner N, Schulmann K, et al. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol. Feb 2010;8(2):174–82. doi: 10.1016/j.cgh.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 8.de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology. Mar 2006;130(3):665–71. doi: 10.1053/j.gastro.2005.11.032 [DOI] [PubMed] [Google Scholar]
- 9.Center for Disease Control and Prevention: Tier 1 Genomic Applications Toolkit for Public Health Departments. Accessed 12/9/2019, 2019. https://www.cdc.gov/genomics/implementation/toolkit/ [Google Scholar]
- 10.Blandy C, Chabal F, Stoppa-Lyonnet D, Julian-Reynier C. Testing participation in BRCA1/2-positive families: initiator role of index cases. Genet Test. 2003;7(3):225–33. doi: 10.1089/109065703322537241 [DOI] [PubMed] [Google Scholar]
- 11.Fehniger J, Lin F, Beattie MS, Joseph G, Kaplan C. Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns. Oct 2013;22(5):603–12. doi: 10.1007/s10897-013-9592-4 [DOI] [PubMed] [Google Scholar]
- 12.Katapodi MC, Viassolo V, Caiata-Zufferey M, et al. Cancer Predisposition Cascade Screening for Hereditary Breast/Ovarian Cancer and Lynch Syndromes in Switzerland: Study Protocol. JMIR Res Protoc. Sep 2017;6(9):e184. doi: 10.2196/resprot.8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suthers GK, Armstrong J, McCormack J, Trott D. Letting the family know: balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet. Aug 2006;43(8):665–70. doi: 10.1136/jmg.2005.039172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharaf RN, Myer P, Stave CD, Diamond LC, Ladabaum U. Uptake of genetic testing by relatives of lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. Sep 2013;11(9):1093–100. doi: 10.1016/j.cgh.2013.04.044 [DOI] [PubMed] [Google Scholar]
- 15.Frey MK, Kahn RM, Chapman-Davis E, et al. Prospective Feasibility Trial of a Novel Strategy of Facilitated Cascade Genetic Testing Using Telephone Counseling. J Clin Oncol. Jan 2020:JCO1902005. doi: 10.1200/JCO.19.02005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frey MK, Ahsan MD, Bergeron H, et al. Cascade testing for hereditary cancer syndromes: Should we move towards direct relative contact? A systematic review and meta-analysis. Journal of Clinical Oncology (In Press) 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Cancer. Version 2.2021. 2020. [Google Scholar]
- 18.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines Version 3.2019): Genetic/Familial High-Risk Assessment: Colorectal. Accessed 12/17/2019. [Google Scholar]
- 19.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. Jun 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 20.Holmes-Rovner M, Kroll J, Schmitt N, et al. Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Making. 1996 Jan-Mar 1996;16(1):58–64. doi: 10.1177/0272989X9601600114 [DOI] [PubMed] [Google Scholar]
- 21.Cella D, Hughes C, Peterman A, et al. A brief assessment of concerns associated with genetic testing for cancer: the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. Nov 2002;21(6):564–72. [PubMed] [Google Scholar]
- 22.Hu B, Boselli D, Pye LM, et al. Equal access to care and nurse navigation leads to equitable outcomes for minorities with aggressive large B-cell lymphoma. Cancer. Nov 01 2021;127(21):3991–3997. doi: 10.1002/cncr.33779 [DOI] [PubMed] [Google Scholar]
- 23.Kline RM, Rocque GB, Rohan EA, et al. Patient Navigation in Cancer: The Business Case to Support Clinical Needs. J Oncol Pract. November 2019;15(11):585–590. doi: 10.1200/JOP.19.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner EH, Ludman EJ, Aiello Bowles EJ, et al. Nurse navigators in early cancer care: a randomized, controlled trial. J Clin Oncol. Jan 01 2014;32(1):12–8. doi: 10.1200/JCO.2013.51.7359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manchanda R, Legood R, Burnell M, et al. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi jewish women compared with family history-based testing. J Natl Cancer Inst. Jan 2015;107(1):380. doi: 10.1093/jnci/dju380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reichelt JG, Møller P, Heimdal K, Dahl AA. Psychological and cancer-specific distress at 18 months post-testing in women with demonstrated BRCA1 mutations for hereditary breast/ovarian cancer. Fam Cancer. 2008;7(3):245–54. doi: 10.1007/s10689-008-9182-z [DOI] [PubMed] [Google Scholar]
- 27.Zilliacus E, Meiser B, Gleeson M, et al. Are we being overly cautious? A qualitative inquiry into the experiences and perceptions of treatment-focused germline BRCA genetic testing amongst women recently diagnosed with breast cancer. Support Care Cancer. Nov 2012;20(11):2949–58. doi: 10.1007/s00520-012-1427-6 [DOI] [PubMed] [Google Scholar]
- 28.Schlich-Bakker KJ, ten Kroode HF, Ausems MG. A literature review of the psychological impact of genetic testing on breast cancer patients. Patient Educ Couns. Jul 2006;62(1):13–20. doi: 10.1016/j.pec.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 29.Ringwald J, Wochnowski C, Bosse K, et al. Psychological Distress, Anxiety, and Depression of Cancer-Affected BRCA1/2 Mutation Carriers: a Systematic Review. J Genet Couns. October 2016;25(5):880–91. doi: 10.1007/s10897-016-9949-6 [DOI] [PubMed] [Google Scholar]
- 30.Mushlin AI. To (genetic) test or not to test, that is the question. J Comp Eff Res. Sep 2015;4(5):429–31. doi: 10.2217/cer.15.35 [DOI] [PubMed] [Google Scholar]
- 31.Rief W, Broadbent E. Explaining medically unexplained symptoms-models and mechanisms. Clin Psychol Rev. Oct 2007;27(7):821–41. doi: 10.1016/j.cpr.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 32.Collins VR, Meiser B, Ukoumunne OC, Gaff C, St John DJ, Halliday JL. The impact of predictive genetic testing for hereditary nonpolyposis colorectal cancer: three years after testing. Genet Med. May 2007;9(5):290–7. doi: 10.1097/gim.0b013e31804b45db [DOI] [PubMed] [Google Scholar]
- 33.Aktan-Collan K, Mecklin JP, Järvinen H, et al. Predictive genetic testing for hereditary non-polyposis colorectal cancer: uptake and long-term satisfaction. Int J Cancer. Jan 20 2000;89(1):44–50. [PubMed] [Google Scholar]
- 34.Aktan-Collan K, Haukkala A, Mecklin JP, Uutela A, Kääriäinen H. Psychological consequences of predictive genetic testing for hereditary non-polyposis colorectal cancer (HNPCC): a prospective follow-up study. Int J Cancer. Aug 15 2001;93(4):608–11. doi: 10.1002/ijc.1372 [DOI] [PubMed] [Google Scholar]
- 35.McCuaig JM, Ferguson SE, Vicus D, et al. Reflex BRCA1 and BRCA2 tumour genetic testing for high-grade serous ovarian cancer: streamlined for clinicians but what do patients think? Hered Cancer Clin Pract. Apr 13 2022;20(1):15. doi: 10.1186/s13053-022-00221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieberman S, Lahad A, Tomer A, et al. Familial communication and cascade testing among relatives of BRCA population screening participants. Genet Med. 11 2018;20(11):1446–1454. doi: 10.1038/gim.2018.26 [DOI] [PubMed] [Google Scholar]
- 37.Menko FH, Jeanson KN, Bleiker EMA, et al. The uptake of predictive DNA testing in 40 families with a pathogenic BRCA1/BRCA2 variant. An evaluation of the proband-mediated procedure. Eur J Hum Genet. August 2020;28(8):1020–1027. doi: 10.1038/s41431-020-0618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodd TL, Reichelt J, Heimdal K, Moller P. Uptake of BRCA1 genetic testing in adult sisters and daughters of known mutation carriers in Norway. J Genet Couns. Oct 2003;12(5):405–17. doi: 10.1023/a:03405 [DOI] [PubMed] [Google Scholar]
- 39.Caswell-Jin JL, Zimmer AD, Stedden W, Kingham KE, Zhou AY, Kurian AW. Cascade Genetic Testing of Relatives for Hereditary Cancer Risk: Results of an Online Initiative. J Natl Cancer Inst. Sep 2018;doi: 10.1093/jnci/djy147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frey MK, Finch A, Kulkarni A, Akbari MR, Chapman-Davis E. Genetic Testing for All: Overcoming Disparities in Ovarian Cancer Genetic Testing. Am Soc Clin Oncol Educ Book. Apr 2022;42:1–12. doi: 10.1200/EDBK_350292 [DOI] [PubMed] [Google Scholar]
- 41.Chapman-Davis E, Zhou ZN, Fields JC, et al. Racial and Ethnic Disparities in Genetic Testing at a Hereditary Breast and Ovarian Cancer Center. J Gen Intern Med. Jul 2020;doi: 10.1007/s11606-020-06064-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. Apr 2005;293(14):1729–36. doi: 10.1001/jama.293.14.1729 [DOI] [PubMed] [Google Scholar]
- 43.Meyer LA, Anderson ME, Lacour RA, et al. Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: missed opportunities. Obstet Gynecol. May 2010;115(5):945–52. doi: 10.1097/AOG.0b013e3181da08d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McBride CM, Pathak S, Johnson CE, et al. Psychosocial factors associated with genetic testing status among African American women with ovarian cancer: Results from the African American Cancer Epidemiology Study. Cancer. Dec 09 2021;doi: 10.1002/cncr.34053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kassem N, Althouse SK, Monahan P, et al. Racial Disparities in Family Variant Testing for Cancer Predisposition Genes. Cancer Epidemiol Biomarkers Prev. Jul 01 2022;31(7):1511. doi: 10.1158/1055-9965.EPI-22-0476 [DOI] [Google Scholar]
- 46.Nitecki R, Moss HA, Watson CH, et al. Facilitated cascade testing (FaCT): a randomized controlled trial. Int J Gynecol Cancer. Dec 2020;doi: 10.1136/ijgc-2020-002118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikolaidis C, Duquette D, Mendelsohn-Victor KE, et al. Disparities in genetic services utilization in a random sample of young breast cancer survivors. Genet Med. June 2019;21(6):1363–1370. doi: 10.1038/s41436-018-0349-1 [DOI] [PubMed] [Google Scholar]
- 48.Sun L, Brentnall A, Patel S, et al. A Cost-effectiveness Analysis of Multigene Testing for All Patients With Breast Cancer. JAMA Oncol. Oct 03 2019;doi: 10.1001/jamaoncol.2019.3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koldehoff A, Danner M, Civello D, Rhiem K, Stock S, Müller D. Cost-Effectiveness of Targeted Genetic Testing for Breast and Ovarian Cancer: A Systematic Review. Value Health. February 2021;24(2):303–312. doi: 10.1016/j.jval.2020.09.016 [DOI] [PubMed] [Google Scholar]
- 50.Tuffaha HW, Mitchell A, Ward RL, et al. Cost-effectiveness analysis of germ-line BRCA testing in women with breast cancer and cascade testing in family members of mutation carriers. Genet Med. Jan 2018;doi: 10.1038/gim.2017.231 [DOI] [PubMed] [Google Scholar]