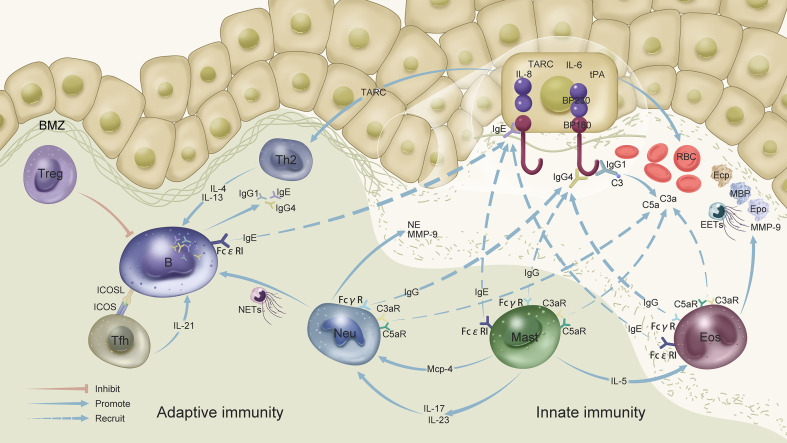
Figure 1.
Innate and adaptive immunity in bullous pemphigoid: B cells produce IgE, IgG1, and IgG4 autoantibodies to bind antigens to the BMZ. Antigen–IgG1 binding to the BMZ triggers complement activation. C3 activation at the dermal-epidermal junction leads to the formation of chemotactic peptides (C3a and C5a), which recruit neutrophils and eosinophils and induce mast cell degranulation, thereby contributing to blister formation. Antigen–IgG4 binding leads to the recruitment of neutrophils and eosinophils and, consequently, to the release of proteolytic enzymes. BP180-specific IgG autoantibodies modulate IL-6, IL-8, and tPA expression in human keratinocytes. TARC/CCL17 secreted by keratinocytes can recruit and activate Th2 cells. IgE autoantibodies amplify the inflammation in BP by interacting with eosinophils, mast cells, and B cells. IgE autoantibodies could also induce BP180 internalization in basal keratinocytes, thereby suppressing their adhesion. Tfh cells promote the production of high-affinity autoantibodies from B cells via regulation by IL-21 and ICOS-ICOSL. Activated Th2 cells secrete IL-4, which regulates IgG isotype and IgE switching. Mast cells activated by IgE degranulate and release IL-5 to promote eosinophil accumulation and activation. Eosinophils are attracted to the BMZ by IgG autoantibodies and complement fixation, and degranulate after interacting with IgE. Eosinophils secrete EETs and toxic granule proteins, including ECP, MBP, EPO, and MMP-9, which are involved in the local inflammatory cascade. In addition, activated mast cells release MCP-4, which activates neutrophils. Activated neutrophils release cytokines and proteases, including NE and MMP-9, which degrade the extracellular matrix and split dermal-epidermal junctions. Neutrophils also release NETs and stimulate autoantibody production by B cells. Neutrophils and mast cells release IL-17 and IL-23, thereby significantly enhancing MMP-9 and NE production by neutrophils. tPA, a component of the plasminogen/plasmin system secreted by keratinocytes, may interact with MMP-9 or NE to promote inflammation. What’s more, tPA, MMP-9, NE and eosinophils all could lead to the activation of coagulation system, inducing thrombotic and bleeding risk of skin. BMZ, basement membrane zone; C3, complement 3; EETs, eosinophil extracellular traps; MMP-9, matrix metalloproteinase-9; NE, neutrophil elastase; NETs, neutrophil extracellular traps; MCP-4, mast cell protease-4; C3a, activated third component of complement; C5a, activated fifth component of complement; C3aRs and C5aRs, C3a and C5a receptors, respectively; RBC, red blood cell; EOS, eosinophil; Th, helper T cell; Tfh, T follicular helper cell; Neu, neutrophil; B, B cell; Mast, mast cell; MBP, major basic protein; ECP, eosinophil cationic protein; EPO, eosinophil peroxidase; TARC, thymus and activation-regulated chemokine; tPA, tissue plasminogen activator.