Abstract
Background and Objectives
To explore the clinical characteristics and HLA associations of patients with anti–leucine-rich glioma-inactivated 1 encephalitis (LGI1E) from a large single center in Israel. Anti-LGI1E is the most commonly diagnosed antibody-associated encephalitic syndrome in adults. Recent studies of various populations reveal significant associations with specific HLA genes. We examined the clinical characteristics and HLA associations of a cohort of Israeli patients.
Methods
Seventeen consecutive patients with anti-LGI1E diagnosed at Tel Aviv Medical Center between the years 2011 and 2018 were included. HLA typing was performed using next-generation sequencing at the tissue typing laboratory of Sheba Medical Center and compared with data from the Ezer Mizion Bone Marrow Donor Registry, containing over 1,000,000 samples.
Results
Our cohort displayed a male predominance and median age at onset in the 7th decade, as previously reported. The most common presenting symptom was seizures. Notably, paroxysmal dizziness spells were significantly more common than previously reported (35%), whereas faciobrachial dystonic seizures were found only in 23%. HLA analysis revealed overrepresentation of DRB1*07:01 (OR: 3.18, CI: 20.9 p < 1.e-5) and DRB1*04:02 (OR: 3.8, CI: 20.1 p < 1.e-5), as well as of the DQ allele DQB1*02:02 (OR: 2.8, CI: 14.2 p < 0.0001) as previously reported. A novel overrepresentation observed among our patients was of the DQB1*03:02 allele (OR: 2.3, CI: 6.9 p < 0.008). In addition, we found DR-DQ associations, among patients with anti-LGI1E, that showed complete or near-complete linkage disequilibrium (LD). By applying LD analysis to an unprecedentedly large control cohort, we were able to show that although in the general population, DQB*03:02 is not fully associated with DRB1*04:02, in the patient population, both alleles are always coupled, suggesting the DRB1*04:02 association to be primary to disease predisposition. In silico predictions performed for the overrepresented DQ alleles reveal them to be strong binders of LGI1-derived peptides, similarly to overrepresented DR alleles. These predictions suggest a possible correlation between peptide binding sites of paired DR-DQ alleles.
Discussion
Our cohort presents distinct immune characteristics with substantially higher overrepresentation of DRB1*04:02 and slightly lower overrepresentation of DQB1*07:01 compared with previous reports implying differences between different populations. DQ-DR interactions found in our cohort may shed additional light on the complex role of immunogenetics in the pathogenesis of anti-LGI1E, implying a possible relevance of certain DQ alleles and DR-DQ interactions.
The leucine-rich glioma-inactivated 1 (LGI1) protein is a synaptic protein secreted by neurons in the CNS. Anti–LGI1-associated autoimmunity can affect the central, peripheral, and autonomic nervous systems. Most patients present with CNS symptoms of cognitive decline, behavioral changes, sleep disturbances, and seizures, which may be generalized or focal.1,2 Patients commonly present with hyponatremia, which may be attributed to dysregulated antidiuretic hormone secretion. Two of the more unique CNS manifestations are the distinctive faciobrachial dystonic seizures (FBDSs),3-5 considered pathognomonic for LGI1 encephalitis, and paroxysmal dizziness spells (PDSs).6
Recent research of the postulated autoimmune nature of leucine-rich glioma-inactivated 1 encephalitis (LGI1E) has expanded to explore genetic factors associated with autoimmunity. Of those, the HLA is the most relevant group.7 Specific HLA alleles have been previously linked to a predisposition for autoimmune neurologic diseases, such as myasthenia gravis (MG) and MS.8,9 Thus, it is not surprising that an expanding body of evidence points to an association between certain alleles of HLA genes and anti-LGI1E.
HLA genes correspond to the major histocompatibility complex (MHC) in humans and encode antigen-presenting proteins on the surface of the cells that are directly involved in immune responses. HLA Class I–restricted recognition by CD8+ T cells leads mainly to a cytotoxic T-cell response, whereas HLA Class II–restricted recognition by CD4+ T cells often leads to a B-cell immune response.
HLA Class II molecules are heterodimers consisting of alpha and beta chains, encoded by 3 different loci, DR, DQ, and DP. These genes are highly polymorphic, and their variability modulates the peptide binding affinity of the expressed HLA Class II molecules. For HLA-DR, variability stems chiefly from alleles encoding the beta chain (DRB), as the alpha subunit is practically invariable.10,11 In contrast, both chains of HLA-DQ and -DP are polymorphic. For HLA-DP, however, only a few allelic combinations are prevalent, resulting in limited variability of DP molecules.12
Recent studies reported associations of anti-LGI1E with certain HLA genes (alleles and haplotypes), including HLA-DRB1*07 and HLA-DRB4 in a Dutch population study,13 DRB1*07:01 and DQB1*02:02 in a Korean population study,14 and DRB1*07:01, DRB1*04:02, DQA1*02:01, and DQB1*02:02 in a Caucasian population study.15-18 However, a study in the Chinese population found a significant variation in common haplotypes pointing to DRB1*03:01 and DQB1*02:01 as the most common susceptible HLA loci.19,20 These inconsistent findings may be explained by different ethnicities as HLA alleles vary among diverse ethnic groups, or they may represent various disease phenotypes yet to be deciphered.
Previous reports on HLA clinical association found DRB1*07:01-negative patients to be younger at disease onset and with a female predominance. Furthermore, a moderate association of DRB1*04:02 with an increased modified Rankin Scale (mRS) score at onset was found.18 Herein, we describe the clinical characteristics and HLA associations of 17 patients with anti-LGI1E encephalitis of heterogeneous ethnicities in a single center in Israel.
Methods
Study Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Tel Aviv Medical Center Institutional Review Board. Written informed consent was obtained from all patients.
Subjects
Seventeen consecutive patients diagnosed with anti-LGI1-associated encephalitis at the Neurological Department at Tel Aviv Sourasky Medical Center between the years 2011 and 2018 were included. Detection of anti-LGI1antibodies in serum and CSF was performed with the EUROIMMUN cell-based assay, as previously described.6 The clinical information, auxiliary diagnostic tests results, and treatment types of patients were obtained from Tel-Aviv Medical Center medical records. Response to treatment was assessed based on the physician and patient reports, Montreal Cognitive Assessment (MoCA) scores, and mRS. An experienced epileptologist classified seizures based on files and EEG reports.
Response to treatment was assessed based on physician and patient reports and the mRS scores. These assessments were performed by 2 neurologists (M.N. and A.G.), and the average of the 2 assessments determined the final score. A MoCA test was added for wider exploration of cognitive outcomes.
HLA Analysis and Protein Binding Prediction
1–2 mL of whole blood was collected at the last follow-up for HLA genotypes. Blood samples were sent to the Tissue Typing Laboratory at Sheba Medical Center for HLA typing.
HLA genotyping was performed by the next-generation sequencing (NGS) method using the NGS-go MX6-1 kit for 6 HLA loci (A, B, C, DRB1, DQB1, and DPB1) and NGSgo-AmpX V2 primer for HLA-DQA1 locus (GenDx, the Netherlands) on Illumina MiniSeq instrument. HLA typing results were obtained with the analysis of sequencing data (FASTQ files) using NGSengine software (GenDx, the Netherlands). The HLA genotypes were imputed to estimate the most probable haplotype pair for each typing for haplotype-based association using GRaph IMputation and Matching for HLA Genotypes.21
The control group was obtained from the Ezer Mizion Bone Marrow Donor Registry, counting over 1,000,000 donors. The haplotype frequencies were estimated using a multirace expectation maximization algorithm22 (MR-EM). The allele frequencies were the marginal frequencies of the haplotypes (i.e., the frequency of an allele was the sum of the frequencies of the haplotypes containing it). The frequencies were estimated for the 2 main origin groups of the patients—Sephardic and Ashkenazi Jews, as estimated from the MR-EM.
We used the full-length sequences of LGI1 from the UniProt database in the FASTA format (accession number O95970). Protein binding prediction to different MHC alleles was assessed via NetMHCIIpan4.1.23 We examined the binding prediction for DQ serotypes to consecutive overlapping peptides with a length of 15 amino acids. We considered rank values ≤1 as strong binders and >1 but ≤3 as medium binders. Graphics were created with BioRender (biorender.com).
Statistical Analysis
The association between each HLA allele and haplotype was tested for each HLA separately using χ2 with p value, CI, and OR calculated. A Bonferroni multiple measurement correction was performed.
The expected count of each allele in the overall population was defined to be the weighted average of the frequency of the same allele in the reference population, using the population composition of the current sample (53% Sephardic and 47% Ashkenazi). The same was performed for the haplotypes.
For alleles with a significant association, linkage disequilibrium (LD) was assessed for each ethnicity. The LD coefficient DAB is defined as the difference between the observed frequency of the combined haplotype AB and the expected probability of both A and B to occur together, assuming that the association between the 2 is completely independent. Normalization of D for the broad range of possible values of frequencies was performed by dividing it by the theoretical maximum difference between the observed and expected haplotype frequencies
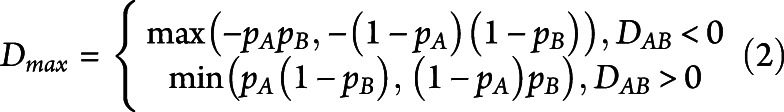 |
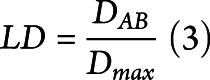 |
Because a higher LD score indicates either higher or lower than random pairing of 2 alleles, when reporting, we multiplied the LD score by −1 in case of lower association (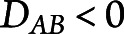 .
.
Data Availability
Data will be shared by request from any qualified investigator.
Results
Epidemiologic and Clinical Features of Patients
Our cohort showed a male preponderance (10 patients, 59%) and a median age at onset in the 7th decade, as previously reported (Tables 1 and 2). Ethnicity showed a rather even distribution between Ashkenazi and Sephardic Jews (8 and 9 patients, respectively).
Table 1.
Patient Characteristics (n = 17)
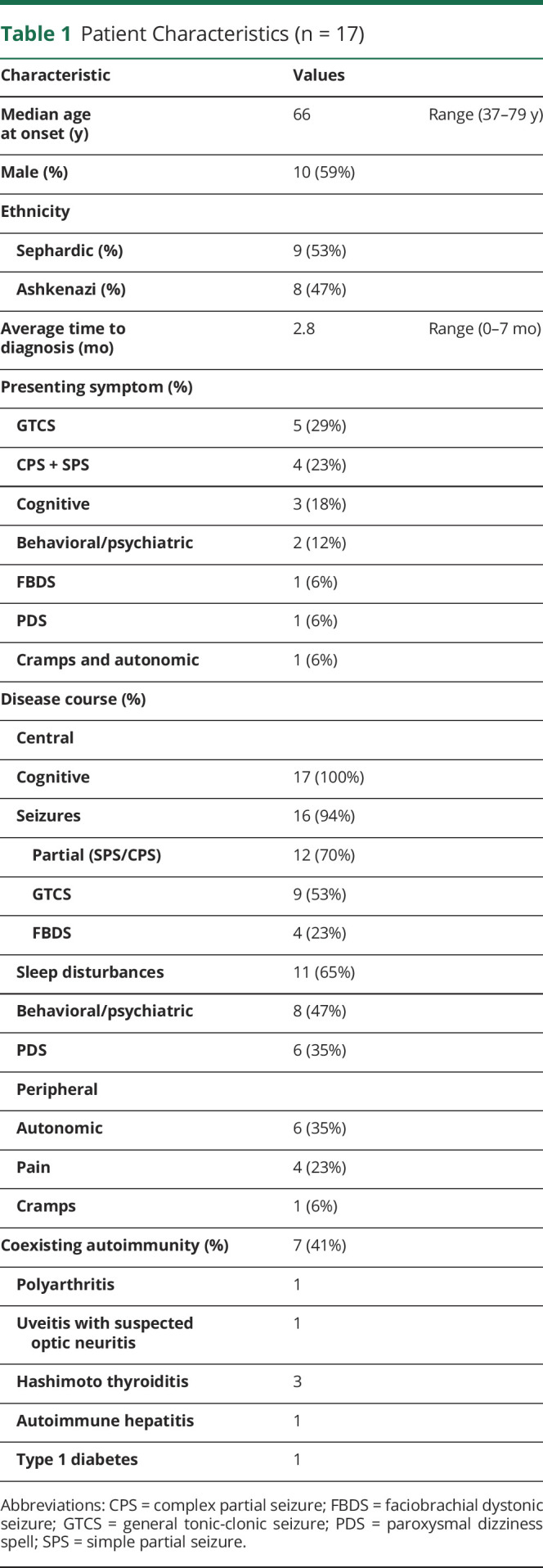
Table 2.
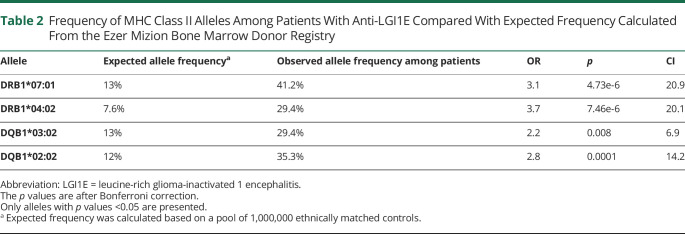
Frequency of MHC Class II Alleles Among Patients With Anti-LGI1E Compared With Expected Frequency Calculated From the Ezer Mizion Bone Marrow Donor Registry
The most common presenting symptom was general tonic-clonic seizures (5 pts, 29%), closely followed by focal seizures with or without impaired awareness (4 pts, 23.5%). Notably, one patient presented with 3 weeks of PDSs (a complaint that does not routinely raise suspicion of encephalitis) before developing a general tonic-clonic seizure. More striking was the fact that only one patient presented with clear FBDS. Throughout the illness, FBDSs were recorded in 4 patients (25%), whereas dizziness spells were observed in 35% (6/17) of patients, significantly higher than previously reported.6
CSF, MRI, and EEG characteristics are further described in eTable 2, links.lww.com/NXI/A815. Of interest, 8/17 (47%) of our patients had another systemic autoimmunity. Most (7/8, 87%) were diagnosed in close proximity to their anti-LGI1E presentation, whereas only one had preexisting hypothyroidism.
The median time from symptom onset to treatment was 2 months, much shorter than what was previously reported in 2017, but in line with more recent reports.24,25 All our patients showed clear improvement following immunomodulatory treatment, although relapses were observed in 29% (5/17) of patients.
HLA Associations Among Patients
We compared our patient population's HLA allele distribution to the distribution of HLA alleles in the Ezer Mizion Bone Marrow Donor Registry, the largest registry of volunteer donors per capita in the world, composed of over 1 million donors. Several distinct alleles were considerably more frequent among patients with anti-LGI1E (Table 3).
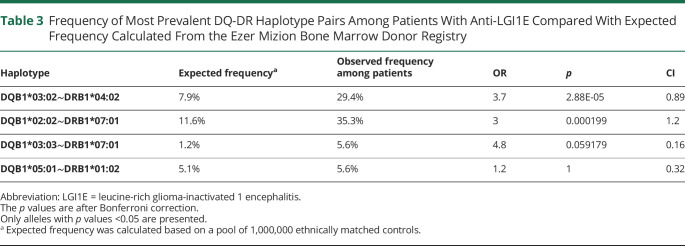
Table 3.
Frequency of Most Prevalent DQ-DR Haplotype Pairs Among Patients With Anti-LGI1E Compared With Expected Frequency Calculated From the Ezer Mizion Bone Marrow Donor Registry
Of those, the most abundant was the HLA-DRB1*07:01 allele, reported previously in several studies.13,14,16,17 It was observed in 12/17 (70%) of our patients (allele frequency being 41% in patients compared with ∼13% in controls, OR: 3.18, CI: 20.9, p value <1E-5); among them, 2 patients (16%) were homozygotes for the allele, whereas the rate of homozygosity among healthy HLA-DRB1*07:01 carriers was 8%. Assessment of the effect of homozygosity for DRB1*07:01 showed the OR for homozygosity among patients to be double that of heterozygotes (OR: 2.11, p value = 0.05, CI: 0.48–9.54). Additional overrepresented alleles were HLA-DQB1*02:02, observed in 10/17 (59%) of our patients (allele frequency being 35% in patients compared with ∼12% in controls OR: 2.8, CI: 14.2, p value < 0.0001).
This is in agreement with previous reports, which argued that their increased prevalence among patients was attributed to being in a substantial (LD) with HLA-DRB1*07:01.14,16-18 Here, HLA-DQB1*02:02 was present in 2 overrepresented haplotypes (eTable 1, links.lww.com/NXI/A815, estimated using imputation): HLA-A*02:01∼B*13:02∼C*06:02∼DQB1*02:02∼DRB1*07:01(OR: 21, CI: 0.08, p value <1.e-8) and HLA-A*02:05∼B*50:01∼C*06:02∼DQB1*02:02∼DRB1*07:01 (OR: 8.36, CI: 0.18, p value <1.e-4).
Further uniquely overrepresented alleles were HLA-DQB1*03:02 and HLA-DRB1*04:02, both observed in 9/17 (53%) of patients (allele frequency being ∼30% in patients compared with 13% in controls. HLA-DQB1*03:02: OR 2.3, CI: 6.94, p value < 0.008, and 8% for HLA-DRB1*04:02: OR: 3.8, CI: 20.1, p value < 1e-5). These 2 alleles appear locked together in complete LD among patients with anti-LGI1E, However, although HLA-DRB1*04:02 is always associated with HLA-DQB1*03:02 in both Sephardic and Ashkenazi background populations, the opposite is not true, and less than 50% of the HLA-DQB1*03:02 are associated with HLA-DRB1*04:02 among these background populations (Table 2 and eTables 3, links.lww.com/NXI/A815). Homozygosity analysis for DRB1*04:02 was not performed due to the low number of homozygote patients. In addition, several Class I alleles were found to be significantly overrepresented among patients with LGI1E patients (eData, links.lww.com/NXI/A815).
Peptide Binding Prediction
Adding to these previous reports regarding the binding affinity of various DR alleles to LGI1 peptides, we aimed to explore the affinity of relevant DQ alleles to such peptides. Previous reports show that DQB1*02:02 and DQB1*03:02 are associated each with a single DQA1 allele (DQA1*02:01 and DQA1*03:02, respectively).26 See eTables 3 and 4, links.lww.com/NXI/A815, for original typing and imputed haplotypes. Using NetMHCIIpan4.1, we assessed peptide binding prediction for each of the overrepresented DQB1 alleles.
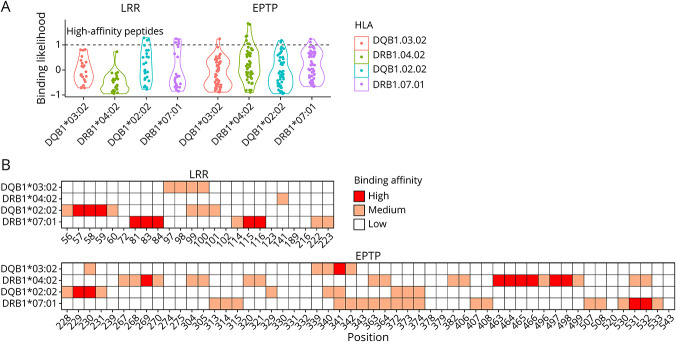
For DQB1*02:02, 18 peptides were predicted as medium/strong binders, encompassing 7 core peptides. The majority of peptides found to be strong binders were located in the leucine-rich repeat (LRR) domain (Figure 1). For DQB1*03:02, 9 peptides were predicted as medium/strong binders, encompassing 4 core peptides. The strongest binder was in the epitempin repeat (EPTP) domain (Figure 1).
Figure 1. Identifying LGI1 Peptides With Predicted High Binding Affinity for Selected HLAs.
(A) Binding likelihood of peptides within epitempin repeat (EPTP) and leucine-rich repeat (LRR) domains to HLA-DQ and HLA-DR molecules. Dashed lines mark the absolute value of the rank binding probability cutoff (=1), where all peptides above the dashed line are ranked strong binders. The likelihood binding was determined by 1-log10(rank). (B) Heatmap of binding affinity of peptides (represented by positions) predicted by NetMHCIIpan4.1 to bind the selected HLAs (high = rank <1, medium = rank 1–3, and low = rank >3). LGI1 = leucine-rich glioma-inactivated 1.
Discussion
We report the clinical phenotype and HLA associations of anti-LGI1E encephalitis patients in a large tertiary center in Israel. The clinical features of our cohort bear a resemblance to previous descriptions in the wide prevalence of seizures and cognitive impairment.27 However, in contrast to previous reports, our patients presented with significantly lower rates of FBDS (reported in 23% of our patients while previously reported in 50%–70%14,16,28). Furthermore, PDSs, were documented in over a third of our patients. Previous accounts of PDSs are limited, with rates ranging from 14% in a study of 200 patients6 to 45% in a report of 20 patients with contactin-associated protein-like 2 encephalitis.29
Such variations in the clinical phenotype may be partially explained by variations in research definitions of clinical symptom categories, as well as the ever-expanding growth of knowledge regarding this relatively novel clinical entity (thus, PDSs were initially reported in association with LGI1E merely 5 years ago). Another cause may be the difference in documentation; PDS is easy to recover from archival files because it is reported by the patients, whereas FBDS may have easily been overlooked by the physician as it was a new entity, therefore not easy to recover from the files since not documented. To strengthen this hypothesis, since 2017, the rate of FBDS among our patients is around 50%.
Nevertheless, another possible explanation relates to underlying genetic properties, which may predispose a patient to develop anti-LGI1E and may attenuate its clinical phenotype. One such possible genetic factor is the HLA gene complex.
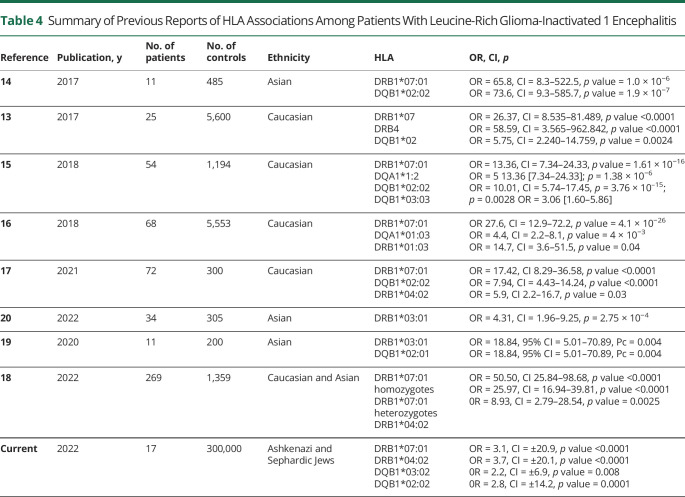
As mentioned, HLA associations with anti-LGI1E were formerly studied among Chinese, Korean, and Caucasian populations with heterogeneous results (Table 4). In accordance with previous research, we examined the HLA genotype of our cohort of patients with anti-LGI1E in search of specific HLA associations that may expand our understanding of disease pathogenesis. Similar to most previous reports, we found DRB1*07:01, DRB1*04:02, and DQB1*02:02 to be significantly overrepresented among patients with anti-LGI1E.
Table 4.
Summary of Previous Reports of HLA Associations Among Patients With Leucine-Rich Glioma-Inactivated 1 Encephalitis
Recently, HLA-DRB1*04:02 was reported18 as independently associated with anti-LGI1E, with a clinical correlation of a younger age at onset. A similarly increased incidence was demonstrated in our cohort, independent of the association to the DRB1*07:01 allele (contrary to previous findings of 78% concordance between the 2 alleles17). In addition to these previously reported alleles, we have recognized DQB1*03:02 as a novel allele associated with anti-LGI1E.
Our findings vary somewhat from previous reports—although previous reports of various populations showed a rate of ∼90% for DRB1*07:01 carriage among patients, our cohorts exhibited a lower rate of 70%. As for DRB1*04:02, while previously reported in 7.4% (20/269) of cases in a large Caucasian cohort, it was found in 53% of our patients. These variations are likely due to the unique ethnic composition in our cohort, which is primarily Jewish, in our case containing about half Sephardic and half Ashkenazi Jews, 2 populations with high DRB1*04:02 frequency (eTable 2B, C, links.lww.com/NXI/A815). It would be of further interest to assess whether such variations in the immunogenetic composition of patients translate to variations on clinical presentation. An additional significant finding of this study in our opinion regards the unique overrepresentation of certain DR-DQ haplotypes among patients and not among controls.
We found the combination of DRB1*04:02 and DQB1*03:02 presented with complete LD among patients (namely all patients presenting with one of the alleles carried the other), whereas analysis of healthy controls reveals a partial co-occurrence p(HLA-DRB1*04:02|HLA-DQB1*03:02) = 0.6. These findings are noteworthy because we examined DR-DQ LD in an unprecedentedly large control cohort of over a million samples, enabling us to discern particular immunogenic features unique to patients with anti-LGI1E. Previous reports of the prevalence of these alleles among the healthy Jewish population, as well as regarding LD analysis in various populations, were based on substantially smaller samples ranging from 100 to 500 subjects.14,30,31
Our data points to these DR-DQ combinations as distinctive immunogenic features of patients with anti-LGI1E and suggests a possible shared role in disease pathogenesis. Such an association to DR-DQ combinations is reported in other autoimmune diseases such as celiac,32 type 1 diabetes,30 and MS31,33
To explore the possible role of DQ alleles in the presentation of LGI1 peptides, we set out to examine protein binding prediction of the relevant DQ alleles to LGI1 peptides. All 3 DQ alleles examined were predicted to bind with high affinity to specific LGI1 peptides.
The binding sites of DQB1*02:02 were relatively evenly distributed between the LRR and EPTP domains. However, the majority of peptides found to be strong binders only were in the LRR domain. In fact, DQB1*02:02 was the only allele predicted to bind with strong affinity to peptides of the LRR domain. The binding sites of DQB1*03:02 were also distributed between the LRR and EPTP domain, yet the strongest binder of DQB1*03:02 was in the EPTP domain.
As demonstrated in eTable 4, links.lww.com/NXI/A815, all patients carrying DQB1*02:02 had DRB1*07:01 as their complementary DRB1 allele, whereas all patients carrying DQB1*03:02 had DRB1*04:02 as their complementary DRB1 allele. Of note, previous reports of binding prediction for DRB1 alleles showed DRB1*07:01 to bind preferentially to the LRR domain, whereas DRB1*04:02 was shown to bind peptides in the EPTP domain.18
An interesting hypothesis may be that among patients carrying DRB1*07:01∼DQB1*02:02, both DR- and DQ-expressed receptors can bind neighboring peptides in the LRR domain of the LGI1 protein, whereas in patients carrying DRB1*04:02∼DQB1*03:02, these receptors can bind adjacent peptides in the EPTP domain (Figure 1). Thus, these DR-DQ combinations unique to patients with anti-LGI1E may take part in the pathogenesis of a distinct immunogenic pathway through specific antigen presentation. As the clinical correlations to specific alleles continue to emerge, the variations in binding targets of the LGI1 protein may serve as an important clue to disease pathogenesis and its relation to clinical phenotypes.
It was previously shown that antibodies binding to the LRR domain trigger internalization of the LGI1-ADAM22 complex, whereas antibodies targeting the EPTP domain interfere with the interaction of LGI1 with ADAM22 or ADAM23.34 Our findings and others suggest that the latter antibodies may be preferentially expressed among carriers of the DRB1*04:02 allele who were reported to present with a younger age at onset.18
Furthermore, the association with DRB1*04:02 may provide an additional clue to disease pathogenesis, as this allele was reported formerly in association with another IgG4-predominant autoimmune disease, Pemphigus Vulgaris, where the role of IgG4 antibodies in disease pathogenesis is known to be through binding a target protein (desmoglein), thus interfering with protein-protein interactions and impairing cell adherence.35
Another avenue of conceivable implications of these findings to disease pathogenesis relates to the DQ8 serotype, represented by the DQB1*03:02 allele. DQ8 was previously linked to several autoimmune diseases, most prominently, type 1 diabetes,36 celiac disease,37 and among the neurologic autoimmune diseases—MG.38
A possible role in disease evolution was eluded by in vitro studies of T-cell lymphocytes from patients with MG carrying various HLA-DQ alleles. T cells from patients carrying the DQ8 serotype (A1*03:01, B1*03:02) were shown to produce an augmented proliferative response following exposure to human acetylcholine receptor peptides.39 This suggests that DQ8 may be associated with a more potent presentation of certain antigens via the MHC Class II receptors, thus leading to the selection and expansion of autoreactive T cells. Notably, patients with MG carrying this haplotype were reported to present with earlier age at disease onset. In fact, the specific combination of DQ8 with certain DR alleles was previously shown to amplify the T-cell proliferative response following exposure to human antigens in a murine model of experimental autoimmune encephalomyelitis.40
In addition to the aforementioned MHC Class II alleles, 3 common haplotypes and 8 Class I alleles were recognized. The significant Class I associations observed in our cohort and others may be explained by LD, as all significant Class I alleles observed in our cohort were present in the 3 common haplotypes containing DRB1*07:01 or DRB1*04:02.
Because of the limited size of our cohort, we were unable to assess HLA clinical associations in our study. Yet, we found it noteworthy that the only patient carrying haplotypes lacking all the MHC Class II disease-specific alleles in ours and previous accounts presented with a distinctive clinical phenotype of cognitive impairment without evidence of any epileptic activity and a striking peripheral involvement. This highlights the need for further investigations of immunogenetic-clinical associations.
Our main study limitation relates to the relatively small cohort of patients and the retrospective nature of clinical data collection. Nevertheless, our study is unique in that allele and haplotype frequencies were compared not with a limited control group but rather with a population-based database, while accounting for our cohort's ethnic distribution (which directly affects certain allele frequencies). This served to reduce confounders, specifically those related to ethnicity, while enabling the recognition of potentially significant associations despite a limited cohort of patients. Most importantly, we revealed significant DR-DQ associations unique to patients with anti-LGI1E, by comparing LD of prominent DR and DQ alleles among our patients to the rates of LD in the general population.
Our findings correspond with novel studies exploring the various epitopes of LGI1 antibodies and their relation to specific mechanisms of pathogenicity.41,42 It was recently suggested that 2 distinct populations of antibodies may exist among patients, with preferential binding to either the LRR or EPTP domain, resulting in internalization of the LGI1-ADAM22/23 complex for the LRR domain, or inhibition of the docking of LGI1 to ADAM22/23 for the EPTP domain.42 The preferential development of these 2 distinct antibodies populations may be related to variations in HLA MHC Class II genetic expression, as eluded by ours and previous studies18 pointing to variations in prediction protein binding sites for different LGI1E-associated genes.
We examined the clinical characteristics and HLA associations of 17 patients with anti-LGI1E. Clinical characteristics were mostly in accordance with previous reports, although the rate of FBDS appeared lower, whereas PDSs were more common among our cohort, and movement disorders were not observed at all. This emphasizes the importance of maintaining a high index of suspicion for this entity in patients of the relevant age group presenting with cognitive and behavioral changes, especially associated with seizures, even without the pathognomonic FBDS.
Analysis of HLA associations revealed, apart from the vastly acknowledged association with HLA-DRB1*07:01, an independent association with HLA-DRB1*04:02 (recently reported) as well as associations with 2 DQ alleles—DQB1*02:02 and DQB1*03:02. These latter alleles appear in significant LD with certain DRB1 alleles among our patients, whereas examination of LD among controls shows a weaker correlation. This suggests that the combination of specific DR and DQ alleles among patients with anti-LGI1E may influence disease pathogenesis. Our findings of similarities in potential binding sites of the LGI1 protein in co-occurring DR and DQ alleles support this possibility.
Notably, our cohort presents distinct immune characteristics with substantially higher overrepresentation of DRB1*04:02 and slightly lower overrepresentation of DQB1*07:01 compared with previous reports. Further studies are required, using large cohorts of ethnically diverse patients with anti-LGI1E, to fully understand this interaction, its possible relation to clinical phenotypes, and its potential clinical implications.
Acknowledgment
The authors thank Ezer Mizion for the data used to produce the frequencies used in this analysis.
Glossary
- EPTP
epitempin repeat
- FBDS
faciobrachial dystonic seizure
- LD
linkage disequilibrium
- LGI1
leucine-rich glioma-inactivated 1
- LGI1E
leucine-rich glioma-inactivated 1 encephalitis
- LRR
leucine-rich repeat
- MHC
major histocompatibility complex
- MoCA
Montreal Cognitive Assessment
- MR-EM
multirace expectation maximization algorithm
- mRS
modified Rankin Scale
- NGS
next-generation sequencing
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133:2734-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai M, Huijbers MGM, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade DM, Tai P, Dalmau J, Wennberg R. Tonic seizures: a diagnostic clue of anti-LGI1 encephalitis? Neurology. 2011;76:1355-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade D, Tai P, Dalmau J, Wennberg R. A common movement abnormality in patients with LGI1 limbic encephalitis is A manifestation of tonic seizures. (P5.246). Neurology. 2014;82. [Google Scholar]
- 5.Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69:892-900. [DOI] [PubMed] [Google Scholar]
- 6.Gadoth A, Pittock SJ, Dubey D, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG-positive patients. Ann Neurol. 2017;82:79-92. [DOI] [PubMed] [Google Scholar]
- 7.Sollid LM, Pos W, Wucherpfennig KW. Molecular mechanisms for contribution of MHC molecules to autoimmune diseases. Curr Opin Immunol. 2014;31:24-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregersen PK, Kosoy R, Lee AT, et al. Risk for myasthenia gravis maps to a (151) Pro→Ala change in TNIP1 and to human leukocyte antigen-B*08. Ann Neurol. 2012;72:927-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Multiple Sclerosis Genetics Consortium, Wellcome Trust Case Control Consortium 2, Sawcer S, Hellenthal G, Pirinen M, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Lith M, McEwen-Smith RM, Benham AM. HLA-DP, HLA-DQ, and HLA-DR have different requirements for invariant chain and HLA-DM. J Biol Chem. 2010;285:40800-40808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang P, Sidney J, Kim Y, et al. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics. 2010;11:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castelli FA, Buhot C, Sanson A, et al. HLA-DP4, the most frequent HLA II molecule, defines a new supertype of peptide-binding specificity. J Immunol. 2002;169:6928-6934. [DOI] [PubMed] [Google Scholar]
- 13.van Sonderen A, Roelen DL, Stoop JA, et al. Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4. Ann Neurol. 2017;81:193-198. [DOI] [PubMed] [Google Scholar]
- 14.Kim T-J, Lee ST, Moon J, et al. Anti-LGI1 encephalitis is associated with unique HLA subtypes. Ann Neurol. 2017;81:183-192. [DOI] [PubMed] [Google Scholar]
- 15.Mueller SH, Farber A, Pruss H, et al. , German Network for Research on Autoimmune Encephalitis GENERATE. Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Ann Neurol. 2018;83:863-869. [DOI] [PubMed] [Google Scholar]
- 16.Binks S, Varley J, Lee W, et al. Distinct HLA associations of LGI1 and CASPR2-antibody diseases. Brain. 2018;141:2263-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñiz-Castrillo S, Haesebaert J, Thomas L, et al. Clinical and prognostic value of immunogenetic characteristics in anti-LGI1 encephalitis. Neurol Neuroimmunol Neuroinflamm. 2021;8:e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peris Sempere V, Muniz-Castrillo S, Ambati A, et al. Human leukocyte antigen association study reveals DRB1*04:02 effects additional to DRB1*07:01 in anti-LGI1 encephalitis. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu F, Liu X, Zhang L, et al. Novel findings of HLA association with anti-LGI1 encephalitis: HLA-DRB1*03:01 and HLA-DQB1*02:01. J Neuroimmunol. 2020;344:577243. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Guo KD, Lin J, et al. HLA-DRB1*03:01 is associated with female sex and younger age of anti-LGI1 encephalitis. Eur J Neurol. 2022;29:2367-2375. [DOI] [PubMed] [Google Scholar]
- 21.Maiers M, Halagan M, Gragert L, et al. GRIMM: GRaph IMputation and matching for HLA genotypes. Bioinformatics. 2019;35:3520-3523. [DOI] [PubMed] [Google Scholar]
- 22.Israeli S, Gragert L, Maiers M, Louzoun Y. HLA haplotype frequency estimation for heterogeneous populations using a graph-based imputation algorithm. Hum Immunol. 2021;82:746-757. [DOI] [PubMed] [Google Scholar]
- 23.Reynisson B, Barra C, Kaabinejadian S, Hildebrand WH, Peters B, Nielsen M. Improved prediction of MHC II antigen presentation through integration and motif deconvolution of mass spectrometry MHC eluted ligand data. J Proteome Res. 2020;19:2304-2315. [DOI] [PubMed] [Google Scholar]
- 24.Qiao S, Wu HK, Liu LL, et al. Clinical features and long-term outcomes of anti-leucine-rich glioma-inactivated 1 encephalitis: a multi-center study. Neuropsychiatr Dis Treat. 2021;17:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson J, Bi M, Murchison AG, et al. , Faciobrachial Dystonic Seizures Study Group. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain. 2018;141:348-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klitz W, Maiers M, Spellman S, et al. New HLA haplotype frequency reference standards: high-resolution and large sample typing of HLA DR-DQ haplotypes in a sample of European Americans. Tissue Antigens. 2003;62:296-307. [DOI] [PubMed] [Google Scholar]
- 27.Ariño H, Armangue T, Petit-Pedrol M, et al. Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology. 2016;87:759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Sonderen A, Thijs RD, Coenders EC, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology. 2016;87:1449-1456. [DOI] [PubMed] [Google Scholar]
- 29.Garrido Sanabria ER, Zahid A, Britton J, et al. CASPR2-IgG-associated autoimmune seizures. Epilepsia. 2022;63:709-722. [DOI] [PubMed] [Google Scholar]
- 30.Kwon OJ, Brautbar C, Weintrob N, et al. Immunogenetics of HLA class II in Israeli Ashkenazi Jewish, Israeli non-Ashkenazi Jewish, and in Israeli Arab IDDM patients. Hum Immunol. 2001;62:85-91. [DOI] [PubMed] [Google Scholar]
- 31.Kwon OJ, Karni A, Israel S, et al. HLA class II susceptibility to multiple sclerosis among Ashkenazi and non-Ashkenazi Jews. Arch Neurol. 1999;56:555-560. [DOI] [PubMed] [Google Scholar]
- 32.Michalski JP, McCombs CC, Arai T, et al. HLA-DR, DQ genotypes of celiac disease patients and healthy subjects from the West of Ireland. Tissue Antigens. 1996;47:127-133. [DOI] [PubMed] [Google Scholar]
- 33.Lincoln MR, Ramagopalan SV, Chao MJ, et al. Epistasis among HLA-DRB1, HLA-DQA1, and HLA-DQB1 loci determines multiple sclerosis susceptibility. Proc Natl Acad Sci U S A. 2009;106:7542-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fels E, Muniz-Castrillo S, Vogrig A, Joubert B, Honnorat J, Pascual O. Role of LGI1 protein in synaptic transmission: from physiology to pathology. Neurobiol Dis. 2021;160:105537. [DOI] [PubMed] [Google Scholar]
- 35.Koneczny I, Yilmaz V, Lazaridis K, et al. Common denominators in the immunobiology of IgG4 autoimmune diseases: what do glomerulonephritis, pemphigus vulgaris, myasthenia gravis, thrombotic thrombocytopenic purpura and autoimmune encephalitis have in common? Front Immunol. 2020;11:605214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. New Engl J Med. 2009;360:1646-1654. [DOI] [PubMed] [Google Scholar]
- 37.Johnson TC, Diamond B, Memeo L, et al. Relationship of HLA-DQ8 and severity of celiac disease: comparison of New York and Parisian cohorts. Clin Gastroenterol Hepatol. 2004;2:888-894. [DOI] [PubMed] [Google Scholar]
- 38.Yang H, Goluszko E, David C, et al. Mapping myasthenia gravis-associated T cell epitopes on human acetylcholine receptors in HLA transgenic mice. J Clin Invest. 2002;109:1111-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deitiker PR, Oshima M, Smith RG, Mosier DR, Atassi MZ. Subtle differences in HLA DQ haplotype-associated presentation of AChR α-chain peptides may suffice to mediate myasthenia gravis. Autoimmunity. 2006;39:277-288. [DOI] [PubMed] [Google Scholar]
- 40.Das P, Drescher KM, Geluk A, Bradley DS, Rodriguez M, David CS. Complementation between specific HLA-DR and HLA-DQ genes in transgenic mice determines susceptibility to experimental autoimmune encephalomyelitis. Hum Immunol. 2000;61:279-289. [DOI] [PubMed] [Google Scholar]
- 41.Kornau H-C, Kreye J, Stumpf A, et al. Human cerebrospinal fluid monoclonal LGI1 autoantibodies increase neuronal excitability. Ann Neurol. 2020;87:405-418. [DOI] [PubMed] [Google Scholar]
- 42.Ramberger M, Berretta A, Tan JMM, et al. Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain. 2020;143:1731-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared by request from any qualified investigator.