Extended Data Fig. 2 |. Voltage dependence of nodal spike propagation failure following dorsal root stimulation.
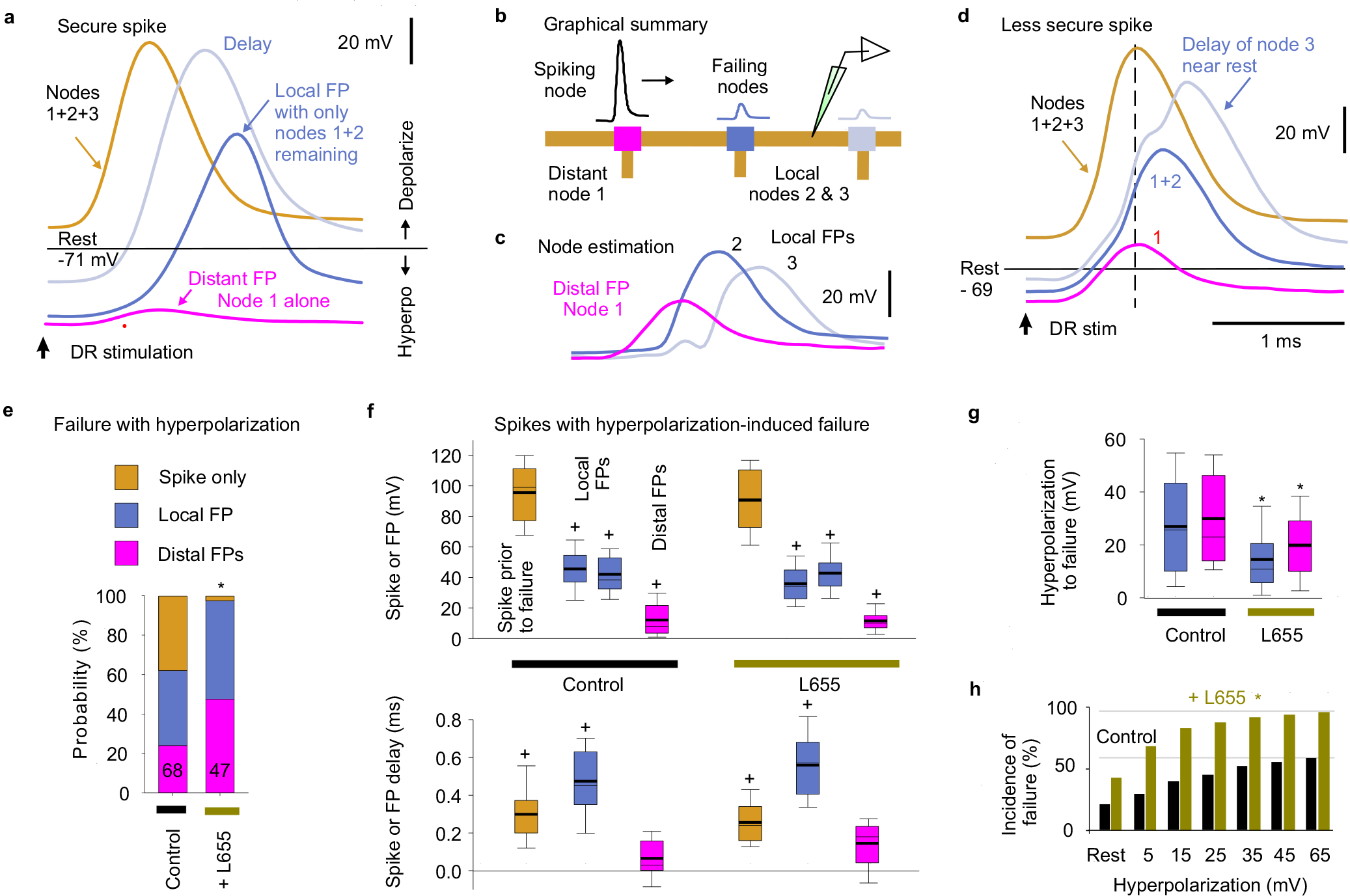
a-d, Intracellular recording from proprioceptive Ia afferent branches in the rat dorsal horn with secure spikes at rest, evoked by DR stimulation (1.1xT, 0.1 ms; T: afferent volley spike threshold; sacral S4 DR; a, d). Spike failure was induced by increasing hyperpolarization (failure near rest in d, but not a), with a delay and then abrupt loss of height, reflecting failure of successively further away nodes (a, d). Of the two local nodes adjacent to the electrode, one failed first with hyperpolarization, leaving the attenuated spike from the other node (local FP, about 1 λS away), which eventually failed as well with further hyperpolarization, leaving a much smaller FP from more distal nodes (distal FP). Spike failure in a node was always proceeded by a delay in the nodal spike. Estimated contributions from local nodes (b-c) were computed by subtraction from traces in (d). Spike attenuation was down to about 1/e by the second failure, consistent with the space constant λS being two internodal distances (c), or about 90 μm. Note that due to attenuation of injected current with distance, larger hyperpolarizations were needed to stop spikes with more distal vulnerable nodes (secure spikes), as observed by smaller distal FPs (a verses d), and some axon spikes could not be stopped (Spike only, quantified in e). Data collected in discontinuous current clamp (DCC) mode so electrode rectification during the injected hyperpolarizing current did not affect potential (DCC switching rate 7 kHz, low pass filtered at 3 kHz to remove switching artifact, which also removed stimulus artifact). e, Distribution of spiking in branches with full spikes only, one local nodal spike (local FP), or a distal nodal spike (distant FP) remaining after maximal hyperpolarization, and * significant change when blocking α5 GABAA receptors with L655708 (0.1 – 0.3 μM) using two-sided χ-squared test, P < 0.05; n = 68 control and n = 47 L655708 treated axon branches from 7 rats. f, Box plots of spike or FP heights and delays in branches and rats indicated in (e), measured just prior to spike failure (or at maximal hyperpolarization for secure spikes) and after failure (for local and distal FPs, as induced in a-d). Delay measured relative to peak of spike at rest. + FP significantly different than spike at rest, n= 7 rats, two-sided paired t-test, P < 0.05. g, Box plots of the hyperpolarization needed to induce failure, in axon branches and rats from (e). * significant change with L655708, two-sided unpaired t-test, P < 0.05, n from (e). Box plots show the interquartile range (box), median (thin line), mean (thick line), extremes, 10 and 90 percentiles (whiskers). h, Incidence of failure at varying potentials (% of total spikes from e). * significantly more failure with L655708, two-sided χ-squared test, n from (e), P < 0.05. Note that secure spikes in rats and mice are not overall different when measure by spike height at rest (Figs. 2g and 3g). However, the spike height shown here in (f) is the height while the cell is hyperpolarized far from rest, and so is much larger than at rest, since spikes generally overshoot to near the reversal potential for sodium, and so spike heights while hyperpolarizing are not comparable to the spikes at rest in mice or rats (measured from holding potential to peak). Thus, during hyperpolarization the nodes produce larger overall spikes, including FPs from local nodes (a-f). Also, during hyperpolarization from current injection, the two adjacent nodes to the electrode fail, unlike during natural failures, and thus when one nodes fails the spike only halves in height, leaving the spike from the second adjacent node (a-b). Thus, these local FPs are large in relation to the FPs from distal nodes with natural failure (Fig 2).
