Keywords: allometery scaling, echocardiography, left ventricular hypertrophy, pediatrics, reference values
Abstract
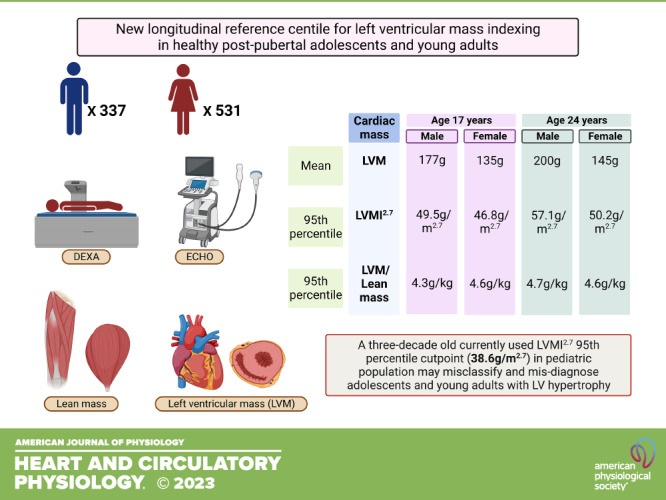
Left ventricular (LV) hypertrophy derived from LV mass (LVM) cut point is a marker of cardiovascular events in adults and target organ damage in pediatric research. Inadequate LVM indexing for body size due to scarcity of dual-energy X-ray absorptiometry (DEXA)-measured lean mass may lead to misclassification in the pediatric population. The only LVM indexed for DEXA-measured lean mass reference in children, mean age 11.6 yr, is 3-decades old and accurate LVM indexing in postpubertal adolescents and young adults is nonexistent. We generate new sex-specific LVM indexed for lean mass percentiles in healthy adolescence and young adulthood and correlated them with surrogates for normalizing body size. From the Avon Longitudinal Study of Parents and Children UK birth cohort, 868 adolescents (531 females) aged 17 yr were followed up for 7 yr. Lean mass was measured by DEXA at both time points. Echocardiography M-mode, two-dimensional (2-D), and three-dimensional (3-D) echo data for estimating LVM were collected at baseline and follow-up. Over 7 years, LVM increased in males (177.1 g) and females (133.5 g) at 17 yr to 199.9 g (males) and 145 g (females) at 24 yr. LVM/height3 and LVM/height2.7 provided the most consistent cross-sectional and longitudinal intraclass correlation coefficients with LVM/lean mass in both sexes (0.90–0.93). Indexing LVM by lean mass eliminated the sex difference only at age 24 yr but not at 17 yr. LVM/height2.7 85th percentiles for males and females at age 17 yr were 45.1 g/m2.7 and 41.4 g/m2.7, respectively, and at age 24 yr the 75th percentiles were 45.5 g/m2.7 and 41.7 g/m2.7, respectively. The 95th percentiles for males and females at age 17 yr were 49.5 g/m2.7 and 46.8 g/m2.7, respectively, and at age 24 yr were 57.1 g/m2.7 and 50.2 g/m2.7, respectively. These new reference percentile cut points were higher than the currently used 95th percentile pediatric reference of 38.6 g/m2.7. Future studies are warranted in youth with clinical diseases to examine whether these new cut points provide a more accurate stratification of cardiovascular risk.
NEW & NOTEWORTHY Current left ventricular mass cut points for pediatric left ventricular hypertrophy are inaccurate. The inaccuracies are due, in part, to the average age of participants (11.6 yr) evaluated and also due to the lack of Echo and DEXA-measured body composition in postpubertal youth. Novel sex-based cut points are proposed for postpubertal youths at 17 and 24 yr. The new 95th percentile cut points are 15–20 g/m2.7 higher than the current cut point.
INTRODUCTION
Among adults, it is well established that left ventricular (LV) hypertrophy is a marker of cardiovascular morbidities and mortality, and guidelines on diagnosis and treatment for pediatric hypertension have used LV hypertrophy as a surrogate for target organ damage (1–3). Seminal studies almost 3 decades ago have proposed LV mass percentiles for identifying LV hypertrophy in the pediatric population (4, 5). LV mass percentiles were derived from LV mass indexed for body composition such as body surface area, height, and lean mass (4, 5). Adult studies have established that lean mass is the strongest predictor of LV mass in both men and women (6, 7) but whether directly measured fat mass or lean mass better predicts LV mass in postpubertal pediatric males and females remains unknown (8, 9). Recent postpubertal pediatric studies reported that males had consistently higher lean mass than females and females had higher fat mass than males over a 7-yr follow-up, which may potentially suggest differing relationships between lean mass, fat mass, and LV mass across sexes (10–12).
LV hypertrophy misclassification in the pediatric population is likely due to the small sample size and mean age of the participants from which previous centiles curves were derived, such as mean age of 11.6 yr in the Daniel et al. study (4) involving 192 children aged 6–17 yr. A new percentile was proposed 6 years ago by Foster et al. (8) in a study involving 1,710 Canadian and American children aged 5–18 yr with a mean age of 12.6 yr. However, the indirect assessment of lean mass derived by equations in the Foster et al.’s study is a major limitation of the LV mass percentiles. Likewise, different echocardiography methods for LV mass assessment and nonassessment of directly measured fat mass in Daniel et al.’s study is a limitation (4, 8). Importantly, these earlier studied mean ages are prepubertal and may not accurately reflect LV mass during postpubertal stages of growth, especially since pediatric youthful population spans until young adulthood (∼24 yr of age) (4, 8). Furthermore, there is a scarcity of longitudinal evidence on the natural development of LV mass in postpubertal adolescents and young adults due to a lack of repeated direct measures of fat mass, lean mass, and LV mass, the previous studies being cross sectional (4, 8).
Therefore, longitudinal LV mass indices derived from various body composition measures validated with a repeated measure of LV mass indexed for lean mass among postpubertal adolescents and young adults were examined using data from the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort (England, UK). It was hypothesized that the average LV mass indices and percentiles will significantly differ from the earlier published pediatric percentiles (4, 8) and that lean mass may be the stronger determinant of LV mass compared with fat mass in both males and females.
METHODS
Study Cohort
Details of the ALSPAC birth cohort have been published earlier (10, 13–15). The ALSPAC birth cohort investigates factors that influence childhood development and growth. Altogether, 14,541 pregnancies from women residing in Avon, Southwestern England, UK, who had a total of 14,676 fetuses, were enrolled between April 1, 1991 and December 31, 1992. When the oldest children were ∼7 yr of age, an attempt was made to bolster the initial sample with eligible cases who had failed to join the study originally, resulting in 913 additional pregnancies. The total sample size for analyses using any data collected after 7 yr of age was 15,454 pregnancies, resulting in 15,589 fetuses. Of these, 14,901 were alive at 1 yr of age. Regular clinic visits of the children commenced at 7 yr of age and are still ongoing. Study data at 24 yr were collected and managed using REDCap electronic data capture tools (16) . For our analysis, we included 868 participants who had complete height, weight, total body fat mass, total body lean mass, and LV mass measures both at baseline (age 17-yr clinic visit) and at follow-up (age 24-yr clinic visit). Excluded participants did not significantly differ in characteristics (data not shown). Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Consent for biological samples has been collected in accordance with the Human Tissue Act (2004). Written, informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time.
Anthropometric and Body Composition
At 17-yr clinic visit, participant height (in m) was measured using a stadiometer (SECA 213, Birmingham, UK) and weight (in kg) using electronic weighing scales (Marsden M-110, Rotherham, UK) (10–12). At 24-yr clinic visit, standing height to the nearest millimeters was measured using a Harpenden wall-mounted stadiometer (Holtain, Ltd, Crosswell, Crymych, UK) and weight to the nearest 0.1 kg at age 24 yr was measured using Tanita TBF-401 (Model A, Tanita Corp., Tokyo, Japan) electronic body composition scales (10–12). Body mass index was computed as weight in kilograms per height in meters squared. Body surface area (BSA) was calculated using the Mosteller equation: ([height (m) × weight (kg)]/36) (5). Total body fat mass and lean mass were measured using DEXA (Lunar Prodigy software version 15, GE Medical Systems, Madison, Wisconsin) at 17- and 24-yr clinic visits. Repeated DEXA measurements for 122 participants were performed on the same day, and the repeatability coefficient (twice the standard deviation of the difference between measurement occasions) for fat mass was 0.5 kg (10–12). Participants were excluded from DEXA scan if they were pregnant, had a radiological investigation using contrast media within the week before the scan, had a recent nuclear medicine investigation with persistent radioactivity, and weight greater than 159 kg. All participants at baseline, age 17-yr clinic visit had attained puberty (10–12, 17).
Cardiac Structure and Function Measures
At 17 yr, echocardiography was performed according to American Society of Echocardiography guidelines (18, 19) by one of the two experienced echocardiographers using an HDI 5000 ultrasound machine (Phillips Healthcare, Amsterdam, The Netherlands) equipped with a P4‐2 Phased Array ultrasound transducer. At 24 yr, echocardiography was performed by two experienced echocardiographers using a Philips EPIQ 7 G Ultrasound System equipped with a X5-1 transducer. Philips Q-station was used for the M-mode, two-dimensional (2-D), and Doppler echo analyses, while TomTec software was used for the three-dimensional (3-D) echo analyses. The reproducibility of echocardiographic examinations was assessed by recalling 30 participants and repeating their measurements. The intraclass correlation of repeated measurements ranged from 0.75 to 0.93 (intraobserver) and 0.78 to 0.93 (interobserver) (20). LV mass (in g) was computed using the Troy formula (21) from LV internal diameter, posterior wall thickness, and interventricular septum thickness, measured during diastole.
Statistical Analysis
Participant’s body composition characteristics were summarized as means and standard deviation. We assessed the normality of variables and explored sex differences using independent t tests. We examined the body composition univariate determinant of LV mass at baseline and follow-up in both cross-sectional and longitudinal analyses. To assess the reliability and absolute agreement of different LV mass indexes, intraclass correlation coefficients were calculated to compare LV mass indexed by lean mass with other methods of standardization in both cross-sectional and longitudinal analyses. Pearson correlation coefficients were derived relating indexed and unindexed values of LV mass to lean mass and were used to compare the effects of different indexation methods in both cross-sectional and longitudinal analyses. Pediatric research and clinically useful LV mass indexing cut points for determining LVH, 75th, 80th, 85th, 90th, and 95th percentiles, were generated for each sex at baseline and follow-up. We also calculated the intraclass correlation coefficients and Pearson’s correlation comparing LV mass indexed for fat mass and other LV mass indices. We computed a population-specific allometric exponent for LV mass indexed for height at baseline and follow-up using log-linear regression analyses. Differences and associations with a two-sided P value of <0.05 were considered statistically significant with conclusions based on effect estimates and their confidence intervals. Analyses involving a sample of 1000 ALSPAC children at 0.8 statistical power, 0.05 α, and two-sided P value would show a minimum detectable effect size of 0.084 standard deviations if they had relevant exposure for a normally distributed quantitative variable (22). All statistical analyses were performed using SPSS statistics software, Version 27.0 (IBM Corp, Armonk, NY).
RESULTS
Cohort Study Characteristics
Participants were 17 yr old at baseline and 24 yr old at follow-up. There were 337 males and 531 females healthy postpubertal adolescents and young adults used to create a longitudinal LV mass indexed for lean mass percentiles. Males had significantly higher height, weight, lean mass, LV mass, and lower fat mass than females. Other characteristics are summarized in Table 1.
Table 1.
Descriptive characteristics of cohort participants
| 17 Yr |
24 Yr |
|||||
|---|---|---|---|---|---|---|
| Male | Female | P Value | Male | Female | P Value | |
| n | 337 | 531 | 337 | 531 | ||
| Age at clinic visit, yr | 17.66 (0.31) | 17.67 (0.30) | 0.732 | 24.49 (0.52) | 24.45 (0.51) | 0.241 |
| Height, m | 1.79 (0.07) | 1.65 (0.06) | <0.001 | 1.80 (0.07) | 1.66 (0.06) | <0.001 |
| Weight, kg | 70.03 (11.07) | 61.51 (10.69) | <0.001 | 77.63 (12.65) | 66.99 (13.66) | <0.001 |
| Body surface area, m2 | 1.86 (0.16) | 1.67 (0.16) | <0.001 | 1.97 (0.18) | 1.75 (0.18) | <0.001 |
| Body mass index, kg/m2 | 21.83 (3.09) | 22.59 (3.64) | 0.002 | 23.88 (3.47) | 24.39 (4.74) | 0.093 |
| Lean mass, kg | 54.57 (5.88) | 37.81 (3.93) | <0.001 | 55.99 (7.08) | 41.04 (5.18) | <0.001 |
| Total fat mass, kg | 12.34 (7.81) | 20.74 (8.31) | <0.001 | 18.78 (7.80) | 23.84 (9.75) | <0.001 |
| LVM, g | 177.13 (39.43) | 133.49 (27.78) | <0.001 | 199.93 (46.09) | 144.97 (33.24) | <0.001 |
| LVMI for body surface area, g/m2 | 94.74 (17.44) | 79.59 (13.59) | <0.001 | 101.28 (19.24) | 82.78 (16.54) | <0.001 |
| LVMI for lean mass, g/kg | 3.24 (0.62) | 3.53 (0.61) | <0.001 | 3.56 (0.62) | 3.53 (0.66) | 0.472 |
| LVMI for fat mass, g/kg | 18.78 (9.39) | 7.22 (2.64) | <0.001 | 12.15 (5.37) | 6.79 (2.56) | <0.001 |
| LVMI for height, g/m | 98.82 (21.11) | 80.85 (16.09) | <0.001 | 110.76 (24.07) | 87.43 (19.64) | <0.001 |
| LVMI for height2, g/m2 | 55.21 (11.66) | 49.03 (9.60) | <0.001 | 61.45 (12.96) | 52.79 (11.92) | <0.001 |
| LVMI for height2.7, g/m2.7 | 36.77 (7.86) | 34.57 (6.82) | <0.001 | 40.72 (8.59) | 37.11 (8.54) | <0.001 |
| LVMI for height3, g/m3 | 30.89 (6.67) | 29.77 (5.92) | 0.010 | 34.14 (7.24) | 31.91 (7.43) | <0.001 |
| LVMI for height0.82 or 0.58, g/m0.82 or 0.58* | 109.76 (23.57) | 88.48 (17.72) | <0.001 | 141.92 (31.51) | 108.10 (24.42) | <0.001 |
| LVMI for weight, g/kg | 2.53 (0.45) | 2.19 (0.40) | <0.001 | 2.59 (0.48) | 2.20 (0.48) | <0.001 |
Values are means (SD); n, number of participants. *Avon Longitudinal Study of Parents and Children (ALSPAC) population-specific indexed height 0.82 for left ventricular mass (LVM) indexed (LVMI) measures at 17 yr and 0.58 for LVMI measures at 24 yr. Differences between sexes were tested using Student’s t test. P < 0.05, statistically significant for sex differences.
Lean mass was a better predictor of LV mass than fat mass in males at ages 17, 24, and 17–24 yr (Table 2). However, fat mass was a better predictor of LV mass than lean mass in females at ages 17, 24, and 17–24 yr (Table 2). LV mass has a near perfect longitudinal regression lines with lean mass in kg or Z-score compared with other body composition measures (data not shown).
Table 2.
Cross-sectional and longitudinal associations of body composition at 17 or 24 yr with LVM at 17 or 24 yr
| 17 Yr |
24 Yr |
|||||||
|---|---|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
|||||
| β (CI) | P Value | β (CI) | P Value | β (CI) | P Value | β (CI) | P Value | |
| n | 337 | 531 | 337 | 531 | ||||
| Height* | 0.220 (0.145–0.295) | <0.001 | 0.269 (0.198–0.339) | <0.001 | 0.279 (0.204–0.354) | <0.001 | 0.211 (0.140–0.282) | <0.001 |
| Weight* | 0.561 (0.479–0.643) | <0.001 | 0.741 (0.650–0.832) | <0.001 | 0.510 (0.429–0.590) | <0.001 | 0.715 (0.616–0.814) | <0.001 |
| Body surface area* | 0.525 (0.450–0.600) | <0.001 | 0.699 (0.616–0.783) | <0.001 | 0.504 (0.429–0.579) | <0.001 | 0.667 (0.578–0.756) | <0.001 |
| Lean mass* | 0.338 (0.283–0.394) | <0.001 | 0.325 (0.284–0.367) | <0.001 | 0.493 (0.429–0.556) | <0.001 | 0.473 (0.420–0.525) | <0.001 |
| Fat mass* | 0.325 (0.240–0.409) | <0.001 | 0.560 (0.461–0.659) | <0.001 | 0.245 (0.158–0.333) | <0.001 | 0.593 (0.478–0.708) | <0.001 |
| Longitudinal associations 17–24 yr | ||||||||
| Height | 0.249 (0.196–0.302) | <0.001 | 0.240 (0.190–0.20) | <0.001 | NA | – | – | – |
| Weight | 0.536 (0.479–0.594) | <0.001 | 0.728 (0.661–0.795) | <0.001 | NA | – | – | – |
| Body surface area | 0.515 (0.462–0.568) | <0.001 | 0.683 (0.622–0.744) | <0.001 | NA | – | – | – |
| Lean mass | 0.412 (0.370–0.455) | <0.001 | 0.398 (0.364–0.431) | <0.001 | NA | – | – | – |
| Fat mass | 0.288 (0.227–0.349) | <0.001 | 0.578 (0.502–0.654) | <0.001 | NA | – | – | – |
Values are standardized regression coefficients (β) and (confidence intervals; CI); n, number of participants. NA, not applicable. *Dependent variables at 17 yr were regressed against left ventricular mass (LVM) at 17 yr; likewise, dependent variables at 24 yr were regressed against LVM at 24 yr. Longitudinal associations of changes in dependent variables from 17 to 24 yr with changes in LVM from 17 to 24 yr.
In the longitudinal analyses, LV mass indexed for lean mass had the highest correlation with LV mass indexed for body surface area in males and females (Table 3). The highest absolute agreement in longitudinal analyses was observed between the LV mass indexed for height2.7 or 3 and LV mass indexed for lean mass among both males and females (Table 3). The reference percentiles for LV mass indexed for various body sizes for males and females at ages 17 and 24 yr, respectively, are shown in Table 4. LV mass indexed for fat mass had the highest correlation and absolute agreement with LV mass indexed for weight in both sexes but particularly in females at baseline and follow-up (data not shown).
Table 3.
Longitudinal Pearson correlation and ICC between standardized LVMI for lean mass 17 to 24 yr and other body composition indexing from 17 to 24 yr
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| Icc | Cronbach’s α | ρ | P Value | Icc | Cronbach’s α | ρ | P Value | |
| n | 337 | 531 | ||||||
| LVMI for body surface area | 0.847 | 0.952 | 0.919 | <0.001 | 0.889 | 0.942 | 0.908 | <0.001 |
| LVMI for fat mass | 0.126 | 0.130 | 0.141 | <0.001 | −0.147 | −0.157 | 0.316 | <0.001 |
| LVMI for height | 0.833 | 0.938 | 0.894 | <0.001 | 0.868 | 0.932 | 0.863 | <0.001 |
| LVMI for height2 | 0.885 | 0.942 | 0.902 | <0.001 | 0.913 | 0.940 | 0.878 | <0.001 |
| LVMI for height2.7 | 0.908 | 0.936 | 0.890 | <0.001 | 0.927 | 0.939 | 0.874 | <0.001 |
| LVMI for height3 | 0.913 | 0.931 | 0.881 | <0.001 | 0.929 | 0.937 | 0.870 | <0.001 |
| LVMI for height0.67* | 0.817 | 0.935 | 0.864 | <0.001 | 0.850 | 0.913 | 0.869 | <0.001 |
| LVMI for weight, g/kg | 0.901 | 0.905 | 0.824 | <0.001 | 0.861 | 0.862 | 0.769 | <0.001 |
*Avon Longitudinal Study of Parents and Children (ALSPAC) population-specific longitudinal indexed height 0.67 for left ventricular mass indexed (LVMI) measures from 17 to 24 yr. n, number of participants. ICC, intraclass correlation. Changes in LVMI for body size from 17 to 24 yr was correlated with changes in LVMI for lean mass from 17 to 24 yr.
Table 4.
Percentile cut points for LVM among males and females at 17 and 24 yr using different body composition indexing
| 17 Yr |
24 Yr |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentile | n | 75th | 80th | 85th | 90th | 95th | 75th | 80th | 85th | 90th | 95th |
| Males | 337 | ||||||||||
| LVM, g | 202.27 | 208.83 | 219.02 | 227.14 | 245.36 | 223.51 | 231.73 | 243.26 | 260.71 | 283.39 | |
| LVMI for body surface area, g/m2 | 105.11 | 108.40 | 112.27 | 118.38 | 123.57 | 112.58 | 115.96 | 121.62 | 127.32 | 137.02 | |
| LVMI for lean mass, g/kg | 3.59 | 3.66 | 3.78 | 3.93 | 4.26 | 3.95 | 4.08 | 4.16 | 4.42 | 4.71 | |
| LVMI for fat mass, g/kg | 24.36 | 26.20 | 28.39 | 32.08 | 37.89 | 14.32 | 15.09 | 16.24 | 19.11 | 22.05 | |
| LVMI for height, g/m | 112.80 | 116.89 | 121.94 | 125.33 | 133.88 | 122.86 | 127.39 | 134.52 | 142.23 | 156.51 | |
| LVMI for height2, g/m2 | 63.70 | 65.12 | 67.48 | 70.43 | 73.57 | 68.52 | 70.93 | 73.38 | 78.95 | 86.32 | |
| LVMI for height2.7, g/m2.7 | 42.10 | 43.58 | 45.11 | 46.72 | 49.48 | 45.46 | 47.32 | 48.79 | 52.80 | 57.12 | |
| LVMI for height3, g/m3 | 35.52 | 36.87 | 37.84 | 39.47 | 41.51 | 38.13 | 39.83 | 41.14 | 44.48 | 47.53 | |
| LVMI for height0.82 or 0.58, g/m0.82 or 0.58* | 124.91 | 129.90 | 135.47 | 139.25 | 148.85 | 157.11 | 163.21 | 172.81 | 185.49 | 199.69 | |
| LVMI for weight, g/kg | 2.80 | 2.91 | 2.94 | 3.06 | 3.25 | 2.91 | 3.00 | 3.07 | 3.24 | 3.25 | |
| Females | 531 | ||||||||||
| LVM, g | 149.90 | 155.05 | 160.57 | 168.45 | 185.36 | 164.72 | 170.23 | 177.63 | 189.43 | 204.54 | |
| LVMI for body surface area, g/m2 | 87.47 | 90.30 | 92.54 | 96.79 | 102.11 | 92.05 | 94.70 | 96.94 | 103.32 | 108.29 | |
| LVMI for lean mass, g/kg | 3.88 | 4.00 | 4.12 | 4.28 | 4.62 | 3.90 | 4.03 | 4.14 | 4.31 | 4.56 | |
| LVMI for fat mass, g/kg | 8.53 | 9.22 | 9.84 | 10.44 | 12.01 | 8.07 | 8.48 | 8.92 | 9.75 | 11.17 | |
| LVMI for height, g/m | 90.37 | 93.18 | 96.77 | 100.57 | 109.98 | 98.73 | 103.11 | 106.65 | 112.46 | 121.49 | |
| LVMI for height2, g/m2 | 54.51 | 55.93 | 58.51 | 61.13 | 65.85 | 59.54 | 62.00 | 64.60 | 67.33 | 71.66 | |
| LVMI for height2.7, g/m2.7 | 38.32 | 39.70 | 41.43 | 43.14 | 46.79 | 41.71 | 43.18 | 45.56 | 47.35 | 50.21 | |
| LVMI for height3, g/m3 | 33.00 | 34.21 | 35.49 | 37.21 | 40.47 | 35.98 | 37.20 | 39.23 | 40.73 | 43.87 | |
| LVMI for height0.82 or 0.58, g/m0.82 or 0.58* | 99.82 | 102.33 | 105.70 | 110.21 | 121.15 | 122.43 | 127.21 | 131.62 | 139.87 | 150.92 | |
| LVMI for weight, g/kg | 2.40 | 2.47 | 2.56 | 2.69 | 2.86 | 2.42 | 2.51 | 2.61 | 2.76 | 2.96 | |
*Avon Longitudinal Study of Parents and Children (ALSPAC) population-specific indexed height 0.82 for left ventricular mass (LVM) indexed (LVMI) measures at 17 yr and 0.58 for LVMI measures at 24 yr. n, number of participants.
DISCUSSION
To our knowledge, this study presents the first and largest longitudinal indexing of LV mass with various body size surrogates and directly measured lean mass and fat mass in postpubertal adolescents and young adults. Adult studies have established that lean mass is the strongest body composition predictor of LV mass in both men and women (6, 7). However, the only pediatric LV mass indexed with a direct measure of lean mass reference established 3 decades ago, did not determine whether lean mass or fat mass was a better predictor of LV mass (4). In the present study involving a postpubertal pediatric population followed up until young adulthood, we observed that lean mass was a stronger predictor of LV mass only in males, whereas fat mass better predicts LV mass in females. This sex difference is due to the significant proportion of lean mass in males and fat mass in females, suggesting that LV mass indexed for fat mass percentile in females could better reflect the physiological increase in LV mass during postpubertal growth. In prepubertal and pubertal children, lean mass measured with bioelectric impedance was found to be most predictive of change in LV mass from baseline to 5 years later (9). In a previous study in children and adolescents (23), it was reported that fat mass may contribute less than four times the contribution of lean mass to LV mass, however, we observed that fat mass contributed almost twice the contribution of lean mass to LV mass in postpubertal females over a 7-yr follow-up period.
Height has been the most widely used index of LV mass and cut points derived from these indices classify LV hypertrophy and have been used to predict clinical events in adults and target organ damage in hypertensive pediatric population (1–3). This study confirms that LV mass indexed for height2.7 and 3 provided the best longitudinal agreement with LV mass indexed for lean mass. The intraclass correlation agreement was significantly higher than previously reported (0.90–0.93 vs. 0.71–0.84), probably because the current population is postpubertal with a baseline age of 17.7 yr compared with a mean age of 11.6 yr in the previous study (4). This significant difference in the mean age of study population also reflects the body composition and LV mass characteristics. Thus, the established LV hypertrophy 95th percentile cut points (4) corresponding to 38.6 g/m2.7 widely used in pediatric studies is significantly lower than the 95th percentile cut points for males and females at 17 yr baseline and 24 yr follow-up in our study. The use of the previous cut points may misidentify and overdiagnose more than 20% of the pediatric population. Similarly, the established adult LV hypertrophy cut points (2) of 51 g/m2.7 may underdiagnose a significant proportion of postpubertal males and females. The most accurate LV mass index for height2.7 95th percentile cut points for LV hypertrophy in 17-yr-old males and females is 49.5 g/m2.7 and 46.8 g/m2.7, respectively. However, at age 24 yr, the LV mass index for height2.7 90th percentile cut point in males is 52.8 g/m2.7, which is higher than the adult’s 51 g/m2.7 cut point for LV hypertrophy.
Percentiles derived from small sample size studies with different echocardiography measures of LV mass, estimates of lean mass may underestimate previous reference data (4, 8). A widely cited normative LV mass indexed for height2.7 in the pediatric population concluded that at age >9 yr, values >40 g/m2.7 in girls and >45 g/m2.7 in boys can be considered abnormal (ie, >95th percentile) (24). Importantly, these values correspond to the 85th percentile in males and females at age 17 yr and the 75th percentile at age 24 yr in the present study. Moreover, in the previous study (24) among 151 boys and 103 girls ≥16 yr old, the 95th percentile cut point for LV mass indexed for height2.7 was 40 g/m2.7 and 39.4 g/m2.7, respectively. Taken together specifying an exact percentile cut point in a pediatric population with varying stages of growth and development is misleading (4, 8, 24), therefore, exact values rather than the generally used 95th percentile cut point at different ages would be clinically useful in accurately identifying LV hypertrophy during postpubertal stages of growth. However, future studies examining the relationships between the new percentiles in the present study and clinical outcomes are warranted.
The significant variations in LV mass indexed for different body compositions reinforce their inadequacies as accurate surrogates for LV mass indexed for lean mass. The utility of LV mass indexed for height2.7 or 3 may be handy in the postpubertal pediatric population but no single LV mass indexed for height2.7 or 3 measure can define LV hypertrophy across the postpubertal age range. LV mass indexed for lean mass slightly varied between males and females at 17 yr of age but there were no statistically significant variations at age 24 yr. Similarly, in a cross-sectional study of adult men and women, aged 25 to 74 yr, LV mass indexed for lean mass measured with bioelectric impedance removed gender difference (25). We, therefore, propose comparing postpubertal pediatric participants given LV mass indexed for height2.7 or 3 value with LV mass indexed for lean mass percentiles.
Strength and Limitation
The present study participants were population-representative healthy volunteers participating in an ongoing well-phenotyped prospective birth cohort study (ALSPAC). Repeated and precise gold standard measures of body composition throughout the follow-up period and echocardiography assessments made it possible to fill the knowledge gap in pediatric LV mass indexed for lean mass. A few limitations may include the inability to determine the most appropriate cut points to define pathological LV hypertrophy necessitating further studies with clinical events. The homogeneity of the study population’s race (Caucasian) warrants further studies in other racial backgrounds. Participants with diagnosed essential hypertension and vascular diseases were less than 1% of the total cohort thus comparing the identification of LV hypertrophy using the different normalization approaches would be biased because of lower statistical power, warranting further research in youth with clinical diseases. Finally, the new percentiles may not be applicable to younger pediatric population.
Conclusions
LV mass indexed for height2.7 or 3 had the most absolute agreement with LV mass indexed for lean mass. However, the new reference LV mass indexed for height2.7 95th percentile cut points were higher than the currently used 95th percentile pediatric reference (4, 8, 24), and may provide a more accurate stratification of cardiovascular risk, but further studies are needed in youth with clinically diagnosed disease conditions. The generally adopted principle of using LV mass indexed for height2.7 95th percentile to define pathological LV hypertrophy in pediatric populations may be misleading, especially in a postpubertal population. Fat mass is a better determinant of LV mass than lean mass in postpubertal females and LV mass indexed for fat mass may also be considered in this population group.
DATA AVAILABILITY
The written, informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party maintained public repository. However, data used for this submission can be made available on request to the ALSPAC Executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. The ALSPAC study website contains details of all the data that are available (http://www.bristol.ac.uk/alspac/researchers/our-data/).
GRANTS
The United Kingdom Medical Research Council and Wellcome Grant 217065/Z/19/Z and the University of Bristol provide core support for ALSPAC. A comprehensive list of grant funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). This publication is the work of the author, and A.O.A. will serve as guarantor for the content of this paper. Dr Agbaje’s research group (UndeRstanding FITness and Cardiometabolic Health In Little Darlings: urFIT-child) was funded by the Jenny and Antti Wihuri Foundation Grant 00180006; North Savo Regional And Central Finnish Cultural Foundation Grants 65191835, 00200150, and 00230190; Orion Research Foundation sr, Aarne Koskelo Foundation, Antti and Tyyne Soininen Foundation, Paulo Foundation, Paavo Nurmi Foundation, Yrjö Jahnsson Foundation Grant 20217390; and Finnish Foundation for Cardiovascular Research Grant 220021.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
A.O.A. conceived and designed research; analyzed data; interpreted results of experiments; prepared figures; drafted manuscript; edited and revised manuscript; approved final version of manuscript.
ACKNOWLEDGMENTS
We are extremely grateful to all the families who took part in this study, the midwives for help in recruiting them, and the whole ALSPAC team, including interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
REFERENCES
- 1. Lorell BH, Carabello BA. Left ventricular hypertrophy. Circulation 102: 470–479, 2000. doi: 10.1161/01.CIR.102.4.470. [DOI] [PubMed] [Google Scholar]
- 2. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med 322: 1561–1566, 1990. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 3. Baker-Smith CM, Flinn SK, Flynn JT, Kaelber DC, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM; Subcommittee On Screening And Management Of High BP In Children. Diagnosis, evaluation, and management of high blood pressure in children and adolescents. Pediatrics 142: e20182096, 2018. doi: 10.1542/peds.2018-2096. [DOI] [PubMed] [Google Scholar]
- 4. Daniels SR, Kimball TR, Morrison JA, Khoury P, Meyer RA. Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am J Cardiol 76: 699–701, 1995. doi: 10.1016/s0002-9149(99)80200-8. [DOI] [PubMed] [Google Scholar]
- 5. de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol 25: 1056–1062, 1995. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 6. Bella JN, Devereux RB, Roman MJ, O'Grady MJ, Welty TK, Lee ET, Fabsitz RR, Howard BV. Relations of left ventricular mass to fat-free and adipose body mass: the strong heart study. The Strong Heart Study Investigators. Circulation 98: 2538–2544, 1998. doi: 10.1161/01.cir.98.23.2538. [DOI] [PubMed] [Google Scholar]
- 7. Kuch B, Hense HW, Gneiting B, Döring A, Muscholl M, Bröckel U, Schunkert H. Body composition and prevalence of left ventricular hypertrophy. Circulation 102: 405–410, 2000. doi: 10.1161/01.cir.102.4.405. [DOI] [PubMed] [Google Scholar]
- 8. Foster BJ, Khoury PR, Kimball TR, Mackie AS, Mitsnefes M. New reference centiles for left ventricular mass relative to lean body mass in children. J Am Soc Echocardiogr 29: 441–447.e2, 2016. doi: 10.1016/j.echo.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 9. Janz KF, Dawson JD, Mahoney LT. Predicting heart growth during puberty: The Muscatine Study. Pediatrics 105: E63, 2000.doi: 10.1542/peds.105.5.e63. [DOI] [PubMed] [Google Scholar]
- 10. Agbaje AO, Barker AR, Tuomainen TP. Effects of arterial stiffness and carotid intima- media thickness progression on the risk of overweight/obesity and elevated blood pressure/hypertension: a cross-lagged cohort study. Hypertension 79: 159–169, 2022. doi: 10.1161/HYPERTENSIONAHA.121.18449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agbaje AO, Barker AR, Mitchell GF, Tuomainen TP. Effect of arterial stiffness and carotid intima-media thickness progression on the risk of dysglycemia, insulin resistance, and dyslipidemia: a temporal causal longitudinal study. Hypertension 79: 667–678, 2022. doi: 10.1161/HYPERTENSIONAHA.121.18754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agbaje AO, Barker AR, Tuomainen TP. Cumulative muscle mass and blood pressure but not fat mass drives arterial stiffness and carotid intima-media thickness progression in the young population and is unrelated to vascular organ damage. Hypertens Res 46: 984–999, 2023. doi: 10.1038/s41440-022-01065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, Molloy L, Ness A, Ring S, Davey Smith G. Cohort profile: The ‘Children of the 90s’-the index offspring of the avon longitudinal study of parents and children. Int J Epidemiol 42: 111–127, 2013. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A, Ring S, Nelson SM, Lawlor DA. Cohort profile: the Avon Longitudinal Study of Parents And Children: ALSPAC mothers cohort. Int J Epidemiol 42: 97–110, 2013. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Northstone K, Lewcock M, Groom A, Boyd A, Macleod J, Timpson N, Wells N. The Avon Longitudinal Study of Parents and Children (ALSPAC): an update on the enrolled sample of index children in 2019. Wellcome Open Res 4: 51, 2019. doi: 10.12688/wellcomeopenres.15132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agbaje AO, Lloyd-Jones DM, Magnussen CG, Tuomainen TP. Cumulative dyslipidemia with arterial stiffness and carotid IMT progression in asymptomatic adolescents: a simulated intervention longitudinal study using temporal inverse allocation model. Atherosclerosis 364: 39–48, 2023. doi: 10.1016/j.atherosclerosis.2022.11.011. [DOI] [PubMed] [Google Scholar]
- 18. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29: 277–314, 2016. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28: 1–39.e14, 2015. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 20. Timpka S, Macdonald-Wallis C, Hughes AD, Chaturvedi N, Franks PW, Lawlor DA, Fraser A. Hypertensive disorders of pregnancy and offspring cardiac structure and function in adolescence. J Am Heart Assoc 5: e003906, 2016. doi: 10.1161/JAHA.116.003906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Troy BL, Pombo J, Rackley CE. Measurement of left ventricular wall thickness and mass by echocardiography. Circulation 45: 602–611, 1972. doi: 10.1161/01.cir.45.3.602. [DOI] [PubMed] [Google Scholar]
- 22. Golding G, Pembrey P, Jones J; ALSPAC Study Team. ALSPAC-the Avon Longitudinal Study of Parents and Children I. Study methodology. Paediatr Perinat Epidemiol 15: 74–87, 2001. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 23. Daniels SR, Kimball TR, Morrison JA, Khoury P, Witt S, Meyer RA. Effect of lean body mass, fat mass, blood pressure, and sexual maturation on left ventricular mass in children and adolescents. Statistical, biological, and clinical significance. Circulation 92: 3249–3254, 1995. doi: 10.1161/01.cir.92.11.3249. [DOI] [PubMed] [Google Scholar]
- 24. Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 22: 709–714, 2009. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 25. Hense HW, Gneiting B, Muscholl M, Broeckel U, Kuch B, Doering A, Riegger GA, Schunkert H. The associations of body size and body composition with left ventricular mass: impacts for indexation in adults. J Am Coll Cardiol 32: 451–457, 1998. doi: 10.1016/s0735-1097(98)00240-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The written, informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party maintained public repository. However, data used for this submission can be made available on request to the ALSPAC Executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. The ALSPAC study website contains details of all the data that are available (http://www.bristol.ac.uk/alspac/researchers/our-data/).


