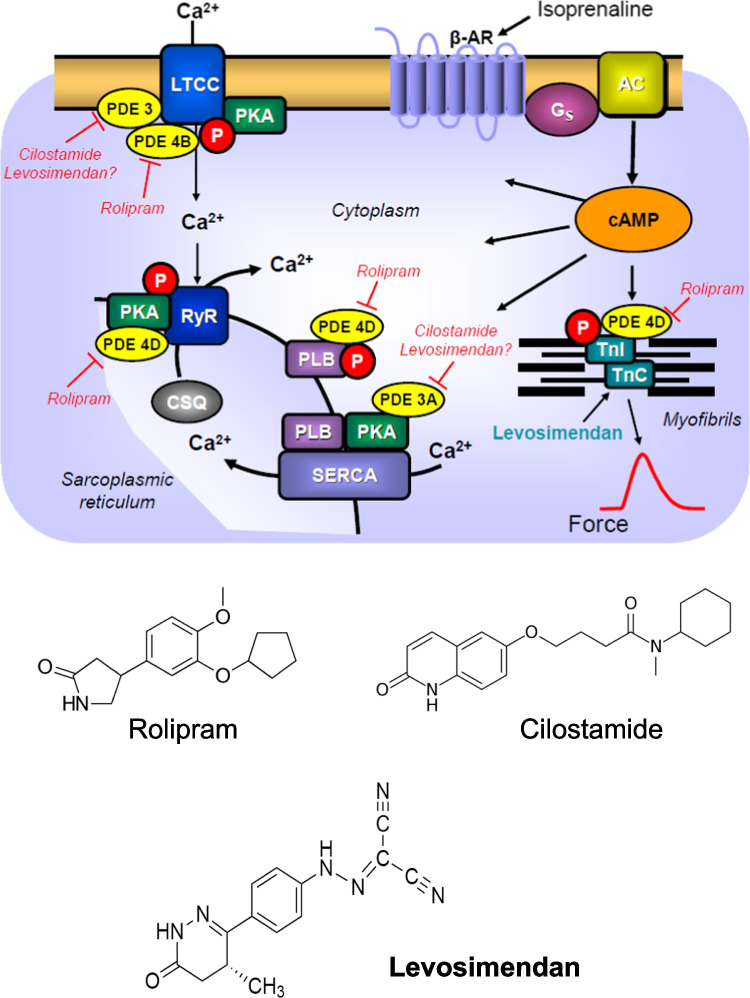
Fig. 1.
Scheme: Potential mechanism(s) of action of levosimendan in the human and mouse cardiomyocytes. Stimulation of the activity of β-adrenoceptors (β-AR) by endogenous noradrenaline or exogenous isoprenaline leads via stimulatory GTP-binding proteins (Gs) to an increase of adenylyl cyclase (AC) activity. Adenylyl cyclase increases the formation of 3′,5′-cyclic adenosine monophosphate (cAMP) that stimulates cAMP-protein kinase (PKA). PKA phosphorylates and thus activates phospholamban (PLB) at the amino acid serine 16, the inhibitory subunit of troponin (TnI), the ryanodine receptor (RYR) and the L-type calcium channel (LTCC). The formed cAMP can be degraded to inactive 5′-AMP and pyrophosphate by isoenzymes of the phosphodiesterase family of proteins (PDE). Cilostamide and rolipram (bottom: structural formulae) inactivate phosphodiesterases 3 and 4, respectively. The β-adrenoceptor is blocked by β-adrenoceptor blockers like timolol or propranolol. Calcium cations (Ca2+) are stored on calsequestrin (CSQ) in the sarcoplasmic reticulum and are released via RYR from the sarcoplasmic reticulum (SR). These released calcium cations bind to troponin C on thin myofilaments, and as a result, systolic force is augmented. Typical calcium sensitizers like EMD57033 act by generating at a given level of free Ca2+ more force of contraction. In cardiac diastole, Ca2+ concentrations fall because Ca2+ is pumped into the SR via the SR calcium ATPase (SERCA). The activity of SERCA is increased when phospholamban is phosphorylated on amino acid serine 16. Levosimendan (bottom: structural formula) might act via increasing the sensitivity for calcium cations of troponin C in the thin myofilaments or might inhibit phosphodiesterases in the heart