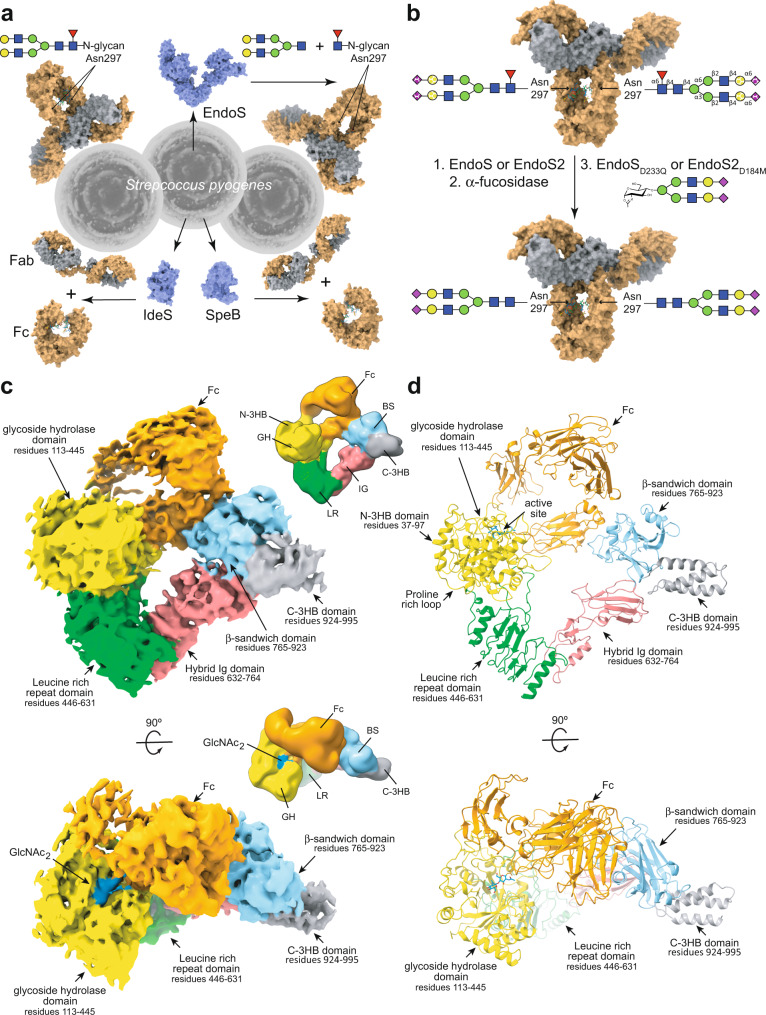
Fig. 1. Overall structure of EndoS in complex with the Fc region.
a EndoS, SpeB and IdeS are secreted enzymes from S. pyogenes that can act on IgG antibodies. SpeB and IdeS are proteases that hydrolyze the flexible hinge region between residues 220 to 248 of the IgG antibody96–98. EndoS is an ENGase that hydrolyze the β−1,4 linkage between the first two GlcNAcs of the CT N-glycans on the Fc region of IgG antibodies. The three enzymes inactivate IgG antibodies against S. pyogenes. b EndoS and EndoS2 can be used to remodel the N-glycan on therapeutic IgG antibodies using a chemoenzymatic approach. First, wild-type EndoS and/or EndoS2 hydrolyze a variety of glycoforms present in the Fc region according to their N-glycan specificity. Next, other enzymes can be used to hydrolyze specific carbohydrate moieties, e.g. fucose which absence improves the binding to FcγRIIIa and the antibody-dependent cellular cytotoxicity (ADCC) properties of the antibody. Last, EndoSD233Q and EndoS2D184M glycosynthase mutants facilitate the transfer of a glycan-oxazoline donor with a defined glycoform to the Fc region of IgG antibodies. The dotted shapes represent that carbohydrate may or may not be present, indicating the high heterogeneity of the N-glycan which impact the functionality, immunogenicity and pharmacokinetic of the antibody. c CryoEM maps of the EndoSE235A-Fc complex, including a schematic representation of the EndoS domains and the Fc region in the complex structure. d Structural model showing the overall fold and the secondary structure organization of the EndoS-Fc complex.