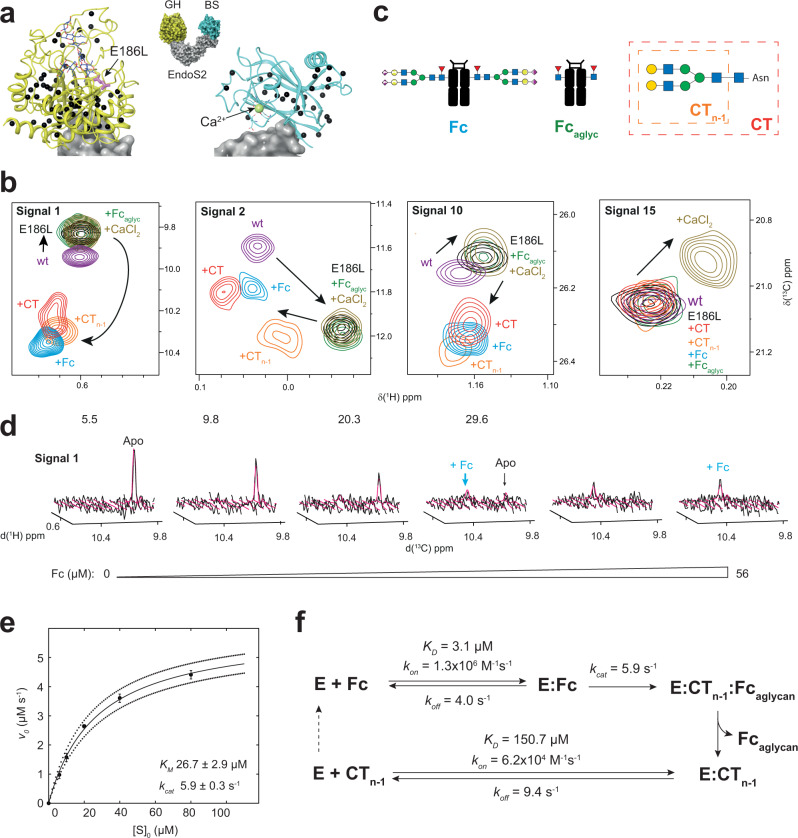
Fig. 6. Binding affinity and enzyme kinetics experiments studied by NMR.
a Crystal structure of EndoS2 bound to CT N-glycan and Ca2+ (PDB code 6MDS) showing the location of the [13C,1H3]-methyl labeled probes as black spheres. Glycoside hydrolase (GH) and β-sandwich (BS) domains are colored in yellow and pale blue, respectively. Mutated amino acid E186L, CT glycan and Ca2+ ion are depicted in pink, dark blue and pale green. b Superimposition of selected regions of methyl-TROSY spectra showing CSPs upon mutagenesis or at saturation concentrations of different ligands. Color code corresponds to: EndoS2 (violet), EndoS2E186L in the absence (black) and in the presence of CT (red), CTn-1 (orange), IgG1 Fc (blue) and Ca2+ (yellow). Addition of Fcaglyc (green) induced to CSPs, suggesting very weak protein-protein interactions. Arrows indicate the extent of CSPs observed between the different protein states. c Ligands used for NMR titration experiments. IgG1 Fc region (Fc), complex-type carbohydrate (CT) and the products of glycan hydrolysis Fcaglyc and CTn−1, respectively. Color code like in Fig. 1. d Quantum mechanics-based 2D lineshape fitting of methyl-TROSY spectra of a representative signal for the titration of EndoS2E186L with Fc. Experimental and fitted spectra are shown as black and pink surfaces, respectively. Inserts correspond to 0, 5.5, 9.8, 20.3, 29.6 and 56 µM Fc concentrations. e [S]0-dependence of the initial enzyme cycling velocity. The solid line represents the best-fit to Michaelis Menten Eq. 4, and dotted lines correspond to one standard deviation. Data are presented as mean, and errors correspond to standard deviation. f Schematic mechanism of Fc N-glycan processing by EndoS2 as determined from lineshape analysis. The affinity of EndoS2 (here E) for Fc is two orders of magnitude higher as compared to the reaction product CTn−1, promoting substrate depletion. On-rates are responsible for increased affinity towards Fc as compared to CTn−1, suggesting differences in the initial enzyme-ligand encounter event. Values correspond to (f) and Table 1. All experiments were acquired at 600 MHz and 298 K.