Abstract
The temperature dependence of infection reflects changes in performance of parasites and hosts. High temperatures often mitigate infection by favoring heat-tolerant hosts over heat-sensitive parasites. Honey bees exhibit endothermic thermoregulation—rare among insects—that can favor resistance to parasites. However, viruses are heavily host-dependent, suggesting that viral infection could be supported—not threatened—by optimum host function. To understand how temperature-driven changes in performance of viruses and hosts shape infection, we compared the temperature dependence of isolated viral enzyme activity, three honey bee traits, and infection of honey bee pupae. Viral enzyme activity varied <2-fold over a > 30 °C interval spanning temperatures typical of ectothermic insects and honey bees. In contrast, honey bee performance peaked at high (≥ 35 °C) temperatures and was highly temperature-sensitive. Although these results suggested that increasing temperature would favor hosts over viruses, the temperature dependence of pupal infection matched that of pupal development, falling only near pupae’s upper thermal limits. Our results reflect the host-dependent nature of viruses, suggesting that infection is accelerated—not curtailed—by optimum host function, contradicting predictions based on relative performance of parasites and hosts, and suggesting tradeoffs between infection resistance and host survival that limit the viability of bee ‘fever’.
Subject terms: Microbial ecology, Ecophysiology, Infection
Deformed Wing Virus best infects honey bee pupae at temperatures optimal for pupal development—not for viral enzymes—suggesting host facilitation of virus replication, and tradeoffs between infection resistance and bee survival.
Introduction
Temperature is a fundamental driver of biological rates and a strong predictor of infection outcome in many systems where host temperature is variable1,2. Metabolic theory provides a framework for explaining the temperature dependence of physiological and ecological phenomena, including host-parasite interactions3–5. This approach uses models derived from enzyme kinetics to describe the temperature dependence of organismal performance (i.e., the ‘thermal performance curve’)6,7. The ‘thermal mismatch hypothesis’ postulates that the outcome of antagonistic bipartite interactions reflects the relative performance of the interacting parties8. Hence, temperature is predicted to impact infection when hosts and parasites respond differently, or in extreme cases, oppositely, over a given temperature range, with parasite growth maximized at temperatures where parasites outperform hosts and minimized where hosts outperform parasites4,9,10. In general, hosts are expected to be most resistant to infection at temperatures within the preferred host range, resulting in lower infection of warm-adapted hosts at warm temperatures. In diverse endo- and ectothermic plants and animals, including insects11–13, infection can be reduced by high body temperatures (i.e., fever12,14–16) that compromise the essential functions of parasites and/or potentiate the immune function of hosts17–19.
The thermal biology of honey bees offers a unique opportunity to study the effects of body temperature on infection. These social bees are endothermic at both the individual and colony level20–22, expending considerable energy to maintain brood temperatures within a remarkably narrow, 34–36 °C range close to mammalian body temperature20,21,23. Honey bee physiology is also highly temperature-sensitive, with peak function achieved within a narrow range of high temperatures. Adults are unable to fly at body temperatures below 30 °C, with muscle force peaking at 38 °C24, and resting metabolic rate increasing 5-fold between 20 and 35 °C25. The immature life stages, which develop entirely within the thermoregulated colony core, are even more temperature-sensitive than are adults. Successful development occurs only between 29 and 37 °C, with mortality and developmental defects occurring outside of the 32–36 °C region and optimal brain development only at 33.5–35 °C26. Nevertheless, honey bees can experience temperatures as low as 5 °C in broodless winter colonies23—although the 20–37 °C range is more typical—and tolerate temperatures of >40 °C during flight22.
Honey bee body temperature has the potential to affect infections by a well-documented assortment of parasites and pathogens, which can adversely affect individual and colony health. These include invertebrate animals, eukaryotic fungi and protozoa, prokaryotic bacteria, and a suite of viruses, many of which also infect less endothermic species27,28. High temperatures achieved by colonies during the brood-rearing season can decrease infection with Varroa mites29, Ascosphaera apis30, Nosema apis and N. ceranae31, and viruses32–34. These results are consistent with mismatched responses to temperature between parasites adapted to diverse ectothermic hosts and endothermic honey bees, which appear to gain a relative advantage over these parasites as temperatures increase towards the peak performance range of bees.
One parasite that has emerged as a formidable honey bee antagonist is Deformed wing virus (DWV). This RNA virus is likely the most prevalent virus among managed bees worldwide35,36. Although historically DWV has not been a major threat to bee health, its virulence has been augmented by the global spread of the Varroa destructor mite, which readily transmits the virus among developing pupae and adult bees, resulting in adults with wing deformities and drastically reduced performance and longevity35. Outside of honey bees, the virus has been found in eight orders of arthropods, including a diversity of wild bees in which its pathogenicity and spillover from honey bees is of potential concern37,38. Yet no antiviral treatments currently exist for honey bee colonies, motivating studies of virus traits, bee defenses, and environmental factors—including temperature—that affect infection39. Adult bees reared at 37 °C show reduced DWV proliferation but also dramatically higher host mortality relative to lower temperatures33. However, effects of temperature on viral infection of susceptible pupae, and the relative contributions of hosts and viruses to such effects, remain unknown. Although more stable than that of honey bee adults, temperature in this life stage can vary by several degrees temporally across the season20, spatially within the brood cluster40, and between female (worker) and male (drone) brood, the latter of which are preferred by virus-vectoring mites;40,41 much greater temperature ranges occur in DWV-associated, non-honey bee host species that do not thermoregulate during development.
The characterization of isolated parasite enzymes offers an opportunity to understand the intrinsic traits of unculturable viruses outside of hosts, while the use of reporter-tagged virus clones allows for controlled inoculations with traceable parasites that can be distinguished from preexisting infections. Both approaches have recently been applied to DWV42,43. Initial evaluation of the first cloned virus enzyme—a protease that cleaves the whole-genome viral polyprotein into individual proteins—showed less than 20% variation in rates of activity across a > 20 °C temperature range43, suggesting that the virus itself is relatively temperature-insensitive. To evaluate the effects of temperature on host-parasite interactions in virus-infected, developing honey bees and the extent to which these dynamics are explained by mismatches between the thermal performance curves of hosts and parasites, we modeled and compared the temperature dependence of performance between a cloned viral protease, three honey bee traits, and pupal infection with a luciferase-tagged clone of DWV.
The preliminary evidence for a contrast between the temperature sensitivity of honey bee physiology—which is optimized at relatively high temperatures—with the relatively constant function of a key viral enzyme across a wide temperature range suggested three alternative hypotheses with divergent predictions for the effects of temperature on infection across the temperature range of the colony: First, if infection is governed primarily by the activity of virus enzymes, then a temperature increase from that typical of an ectothermic insect host to that of an endothermic honey bee colony should have weak effects on infection, reflecting low temperature sensitivity of the virus. Second, if infection is limited by efficient function of the host immune system, then as temperature approaches the high levels over which honey bee metabolism is optimized, infection should rapidly decrease. Third, if rapid proliferation of the virus is instead dependent on strong host performance, then as temperature rises into the range of optimum honey bee function, infection should increase. This differs from the second hypothesis in that the virus is dependent on optimal host function, rather than limited by it, such that infection correlates directly (rather than inversely) with host performance.
Results
Temperature dependence of viral protease activity and honey bee traits
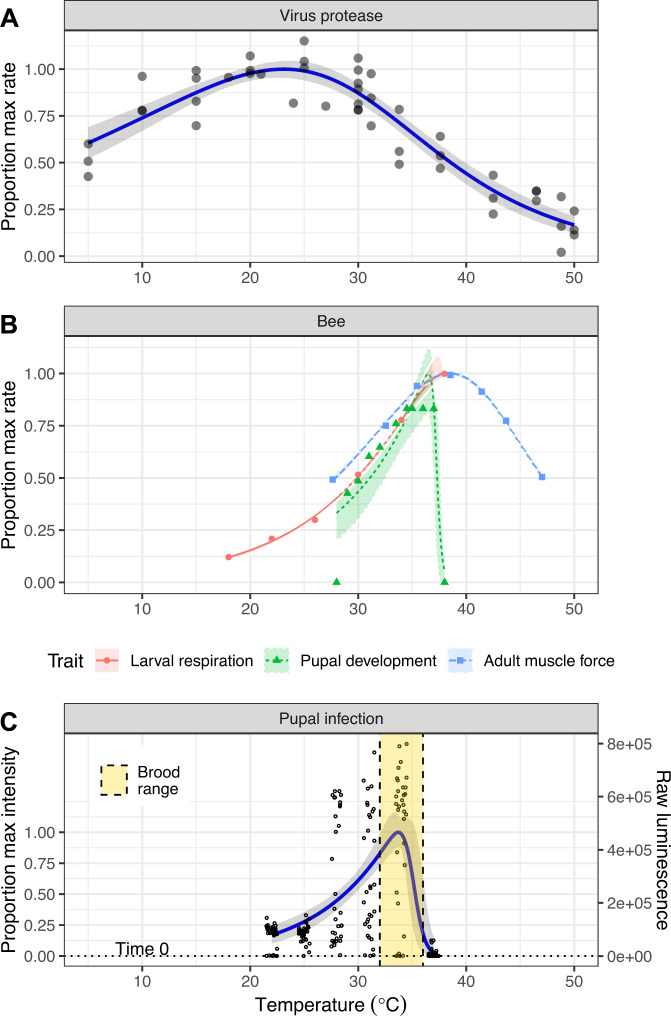
Relative to the bee traits, activity of the viral protease peaked at lower temperatures but was overall less temperature-sensitive. The protease’s broad peak of activity between 15 and 30 °C contrasted with the narrow, 35–40 °C peak for each bee life stage (Fig. 1). The protease’s estimated temperature of 50% inactivation Th (estimate 30.2 ± 2.6 °C SE) was at least 7 °C lower than that of any of the bee traits (40.7 ± 1.8 °C for larval respiration, 37.2 ± 0.58 °C for pupal development, and 40.8 ± 0.62 °C for adult muscle force production (Fig. 2)). The protease’s activation energy e (0.30 ± 0.074 eV), a measure of temperature sensitivity, was also more than two-thirds lower than the average of the three bee traits (0.92 C ± 0.079 eV for larval respiration, 1.06 ± 0.28 eV for pupal development, and 0.83 ± 0.06 eV for adult muscle force production (Fig. 2)). This parameter indicates the extent to which a trait is affected by changes in temperature, with higher values corresponding to stronger temperature-mediated effects. For example, whereas viral protease activity varied <2-fold over a > 30 °C temperature range between 5 and 37.5 °C, larval respiration increased by 8-fold over a 20 °C range (18–38 °C), and adult muscle force production varied by two-fold over just a 10 °C interval (Fig. 1). The pupal stage used for injection is especially sensitive to temperature, with development only completing successfully between 29 and 37 °C (Fig. 1).
Fig. 1. Effects of temperature on Deformed wing virus, honey bees, and infection.
Panels show data (points) and model predictions (lines), and 95% bootstrap confidence intervals (shaded bands) for virus protease activity (A, upper panel), three bee traits (B, middle panel), and pupal infection (C, lower panel). Data for larval respiration (red circles and solid line), pupal development (green triangles and dotted line), adult muscle force production (blue squares and dashed line), and brood temperature range (yellow vertical region in infection panel) taken from previous observations20,24,26,45. In the lower panel, the secondary y-axis indicates the raw sample luminescence (i.e., before transformation to proportion of maximum intensity) and the horizontal line dotted line represents the mean luminescence of n = 16 samples frozen immediately post-injection. To provide a comparable scale for the different measurements, raw values for all traits are shown as proportions of the trait’s peak model-predicted value.
Fig. 2. Heat tolerance and temperature responsiveness of virus protease and bee traits.
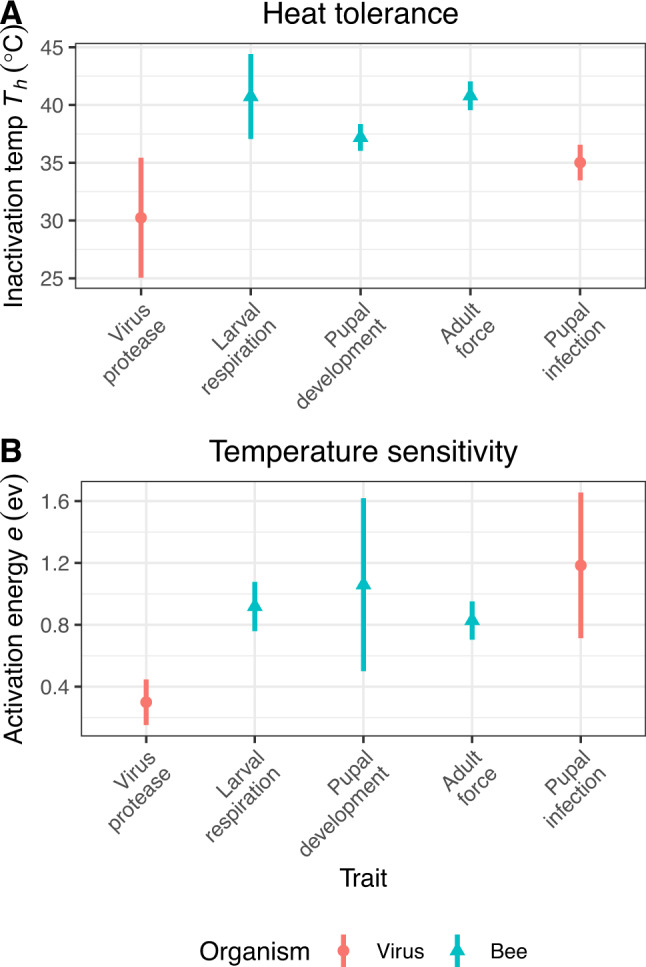
A Temperatures of 50% inactivation (Th) and B activation energies (e) for temperature sensitivity of virus protease (red circles) and bee traits (blue triangles) from Sharpe–Schoolfield models depicted in Fig. 1. Points and error bars show model estimates ±2 standard errors.
The temperature dependence of infection closely reflected that of pupal development, with a temperature-dependent 6-fold increase in infection from 7.66 ∙ 104 ± 7.90 ∙ 103 luminescence units SE at 22 °C to 4.64 ∙ 105 ± 4.54 ∙ 104 units at 34 °C, then a sharp decline (by 98%) to 9.43 ∙ 103 ± 2.91 ∙ 103 SE at 37 °C (Fig. 1). This corresponds to an increase of over ten thousand-fold at the most permissive temperatures relative to the samples taken immediately post-injection (mean 11.9 ± 3.0 SD), indicating successful proliferation of the virus over the 48 h incubation period. The estimated temperature of 50% inactivation was well-defined (35.0 ± 0.76 °C SE) and closest to that of pupae, although confidence intervals overlapped slightly with the broad estimate for the viral protease (Fig. 2). The high temperature sensitivity of infection was reflected by a high estimated activation energy (e = 1.18 ± 0.23 eV SE) much closer to that of the bee traits than the activity of the viral protease (Fig. 2).
Discussion
Our experiments with the DWV protease are qualitatively consistent with this protein’s initial characterization43, indicating a temperature sensitivity (activation energy e < 0.3 eV) at the low end of the usual range observed for biochemical reactions of metabolism (0.6–0.7, range 0.2–1.2 eV44,). The maintenance of protease function at >50% of maximum activity over a > 30 °C range is consistent with infectivity in honey bees across a range of season- and host caste-related temperatures23, and associations of the virus with a wide range of arthropod hosts that exhibit varying degrees of endothermy36. In contrast, the high (>35 °C) temperatures of honey bee peak performance—well above the 25–29 °C reported for most insect traits3—and high thermal sensitivity of honey bee traits (e > 0.9 eV) are consistent with this species’ endothermically controlled body temperature during key periods and activities (e.g., brood development, foraging)45. This control is exemplified by maintenance of temperatures within < 1 °C of 35 °C at the colony core20, site of the highly temperature-sensitive pupal life stage.
Our thermal performance curves of the viral protease and honey bee traits identified a relatively large temperature window—spanning the typical range of colony temperatures—over which viral protease activity declined while honey bee performance rapidly increased. These contrasting responses of host and parasite to temperature allowed us to test the thermal mismatch hypothesis using pupal infection. Specifically, the higher temperature sensitivity and warmer temperatures of peak activity of bee traits relative to the virus enzyme suggested that the relative performance of parasites vs. hosts would decline steeply throughout the range of temperatures typical of the colony, resulting in a corresponding reduction in infection intensity. However, we observed the opposite result, with the temperature dependence of infection—which exhibited a high temperature sensitivity and a well-defined peak in the region of brood incubation temperatures—corresponding to host performance rather than to the relative performance of viruses and hosts. The breakdown in viral proliferation occurred only over the >34.5 °C range where pupal development also becomes threatened, as manifested by decreased emergence success, alteration of brain development, and disruption of subsequent behavior as adults26,46.
Although this result contrasts with predictions based on the expected relative performance of hosts and parasites, it is consistent with a previous study that examined naturally occurring DWV infection in adult bees33, in which a 37 °C rearing temperature resulted in 8- to 17-fold reductions in infection intensity relative to 28 and 34 °C. The latter temperatures also spanned the range of optimal adult survival, the 28 °C preferred honey bee sleeping temperature25, and the 32 °C temperature in which honey bee thermoregulatory energy expenditure is minimized47. This concordance between the performance of hosts and viral infection could reflect the high host dependence of virus production, which exploits the resources and machinery of the host cell, resulting in viral growth rates that are limited by the efficiency of the host as a viral factory. Our results with DWV are also comparable to that of honey bee infection with Nosema spp. microsporidia, which like viruses are intracellular, host energy-dependent pathogens. Infection intensity was increased by 10-fold at 33 °C relative to 25 °C, then decreased by over two orders of magnitude at 37 °C31, mirroring the pattern found here and possibly reflecting niche overlap between these host-dependent parasites, which appear to compete with one another in bees48. Infection with these types of parasites may be less well-predicted by the thermal mismatch hypothesis relative to other parasites that can reproduce independently of host cells9, or whose proliferation is more effectively controlled by a temperature-dependent host immune response.
Despite this host-dependence of intracellular parasites, a match between the temperature dependence of host and parasite performance is certainly not a foregone conclusion; there are numerous counterexamples of arthropod-parasite systems where parasite proliferate fastest at temperatures that are not ideal for hosts. In honey bees, Bee virus X was reported to proliferate in young bees incubated at a below-optimal 30 °C, but not in those incubated at the 35 °C found in their colony34, suggesting a negative relationship between host performance and infection. In mosquito vectors of mammal viruses, infection with most species developed fastest at 37 °C, some 5 °C to 9 °C higher than the optimum temperatures for most of the mosquito traits6. Although these viruses are an exception to the pattern of low infectivity among ectotherm-hosted viruses above 35 °C15,19 —a necessary adaptation for infection of the mammalian bloodstream—they provide additional examples of peak proliferation rates in temperature ranges where insect host physiology is compromised. Hence, their temperature-dependent development in the insect vector is consistent with the predictions of the thermal mismatch hypothesis for performance of a high temperature-adapted parasite in a less heat-tolerant host—i.e., the mammal-hosted viruses are expected to perform well at high, mammal-like temperatures, whereas the mosquito vectors are expected to resist infection most strongly at the lower (near-ambient) temperatures to which these insects are accustomed. Our finding of concordance between host and parasite performance also contrasts with that of another arthropod host-microsporidian parasite system (Daphnia magna and Ordospora colligata), in which parasite growth rate increased over a gradient of temperature that reduced host lifespan by 10-fold (among both parasite-exposed and control hosts)4. Examination of additional virus proteins and processes, and comparison of proliferation in identified hosts36,37 or compatible cell lines39 with different temperature sensitivities and thermal optima, should allow further evaluation of the relationship between host and virus performance and infection with this widespread virus, testing the generality of and examining the mechanisms underlying our result.
Regarding the potential for high colony temperatures to counteract DWV proliferation, although our finding of strongly reduced infection above 34 °C is consistent with high temperature-mediated reductions in insect-, plant-, and bacterium-infecting viruses10,15,19,49,50, the 37 °C upper limit of pupal development suggests either a narrow thermal safety margin or a heavy cost of high temperature-based resistance for hosts. Even in adults, resistance to the virus came at the expense of host survival33, implying that elevation of body temperature up to or beyond 37 °C would not be considered an adaptive “self-medication” response51. Nevertheless, although viral protease function is preserved at higher temperatures than can be tolerated by pupae, it is possible that other key elements of the virus life cycle are disrupted as temperatures approach 37 °C. As honey bees clearly can regulate temperatures between 35 and 37 °C with better than 1 °C precision20,21,23, at least in the brood at the colony center, we cannot rule out a temperature window over which host health is preserved but virus replication is inhibited. It would be of interest to see whether virus inoculation leads to differences in thermal preference, as seen in bees exposed to the similarly heat-sensitive infection with Nosema52.
DWV infects honey bees throughout the year as well as diverse arthropods—including some hosts where infection may be virulent38,53—that exhibit a range of life history strategies and thermoregulatory abilities37. By elucidating factors governing virus replication, including host temperature, this study system has great potential for testing the thermal mismatch hypothesis of host-parasite interactions. Such investigations can evaluate the immunological value of endothermy in predominantly ectothermic host-parasite communities, further elucidating parasite host range, seasonal dynamics, and spillover potential and consequences across seasons, climates, and host communities. This work may also add nuance to the thermal mismatch hypothesis by considering both the parasite-facilitating and -inhibiting aspects of host performance.
Methods
Temperature sensitivity of viral protease activity
We tested the function of a recombinant DWV 3C protease43 (see Supplementary Note 1 for full amino acid sequence) in a fluorescence resonance energy transfer (FRET) assay across temperatures from 5 to 50 °C. The protease (100 nM) was incubated with 80 µM of a fluorogenic DABCYL- VQAKPEMDNPNPG-EDANS-peptide substrate corresponding to the identified target peptide at the interface between the leader protein and VP2 proteins of DWV54 with a buffer consisting of 50 mM Tris [pH 7.0], 150 mM NaCl, 1 mM EDTA, 2 mM DTT, and 10% glycerol (see Supplementary Note 1 for peptide amino acid sequence and reaction buffer composition). The assay was run in temperature-controlled thermocyclers with 50 µL of reaction mixture per well in a standard PCR plate.
We conducted three runs with a temperature range of 5–30 °C (in 5 °C increments) and one run from 15 to 30 °C (in 3 °C increments) using parallel incubation in six thermocyclers, and three runs of eight temperatures each spanning 30–50 °C using the thermocycler’s temperature gradient feature, yielding 48 observations of reaction rate over a 25 °C temperature range. The reaction was sampled at 0, 15, and 30 min of incubation (excluding an initial 5-min equilibration period) by transferring two aliquots per temperature (pooled volume: 80 µL) to a 96-well assay plate, which was read immediately in a Biotek Synergy H1 spectrophotometer (excitation: 340 nm, emission: 520 nm). The maximum rate of increase in fluorescence over either 15-min period was calculated for each sample (n = 1 rate per temperature per run). Net reaction rate was computed by subtracting the maximum rate of increase of a control sample incubated in parallel at the corresponding temperature in the absence of protease, to control for possible differences in spontaneous fluorescence of the substrate across temperatures.
Temperature sensitivity of honey bee traits
To compare the temperature sensitivity of the virus protease to that of bee traits, we gathered data for larval respiration (18–38 °C), pupal development rate (28–38 °C), and adult muscle force production during flight (thoracic temperatures 27.5–47 °C) from existing literature24,26,45. Briefly, larval respiration was measured by CO2 production (µL mg−1 h−1) of 3.5-day-old larvae over a 20 min time interval in 4 °C increments from 18 to 38 °C45. Pupal development rate (d−1) was measured by incubating combs of freshly capped brood in 1 °C increments from 28 to 38 °C and recording the number of days until adult bees emerged26. Adult force production (mN) was recorded from flight muscles of tethered bees at 7 thoracic temperatures from 27.5–47 °C24. We elected not to focus on adult respiration rates because even in isolation, bees may be endothermic at low temperatures and become agitated at high temperature, making it difficult to estimate true resting metabolic rates55.
Temperature sensitivity of virus infection in honey bee pupae
To examine the temperature sensitivity of pupal infection, we used a nanoluciferase-tagged reporter clone of DWV previously developed in our laboratory42. Infection with this reporter clone can be rapidly quantified by luminescence—which has a nearly 1:1 correlation with virus copy number over four orders of magnitude (slope = 0.98, r2 = 0.96)—using a spectrophotometer42. Purple-eyed pupae were removed from brood frames using scalpel and forceps. We injected 32 pupae per temperature with 107 virus particles of reporter-tagged virus-containing inoculum in 4.0 µL of sterile phosphate buffer saline (PBS). Pupae were incubated at six temperatures from 22 to 37 °C (3 °C increments). We chose this temperature range because it spans the typical temperature range experienced by bee brood and adults21 and corresponds to the region where increases in temperature have opposite effects on viral protease activity and bee performance (i.e., protease activity decreases while bee performance increases), suggesting that small changes in temperature could have large and biologically relevant effects on infection.
Injected pupae were incubated for 48 h on filter paper in covered petri dishes. Incubators were manually humidified by placing shallow, water-filled containers on the bottom. After 48 h, pupae were frozen at −80 °C. For luciferase activity-based quantification of infection intensity, samples were individually homogenized for 30 s with glass microbeads in 100 µL PBS. A 5 µL aliquot of homogenate was diluted 10-fold in PBS in a black 96-well microplate. To each sample, we added 50 µL of 2x luciferase substrate-containing buffer from a commercial kit (Promega, Madison, WI). Total luminescence was measured immediately using a spectrophotometer (100 ms integration time). The raw luminescence value was used as the response variable to model the effects of temperature on infection intensity. Sixteen samples frozen immediately post-injection were assayed in parallel to estimate virus replication.
Estimation of thermal performance curves
The effects of temperature on each trait (i.e., protease activity, each bee trait (larval respiration, pupal development, and adult force production), and pupal infection) were modeled using a Sharpe–Schoolfield equation modified for high temperatures7,10,56.
| 1 |
In Eq. (1), rate refers to the observed response variable; rTref is the rate at the calibration temperature Tref (293 K, i.e., 20 °C); e is the activation energy (in eV), which primarily affects the upward slope of the thermal performance curve (i.e., sensitivity of the trait to temperature) at suboptimal temperatures; k is Boltzmann’s constant (8.62 ∙ 10−5 eV∙K−1); eh is the deactivation energy (in eV), which determines how rapidly the thermal performance curve decreases at temperatures above the temperature of peak rate; Th is the high temperature (in K) at which rate is reduced by 50% (relative to the value predicted by the Arrhenius equation—which assumes a monotonic, temperature-dependent increase);7 and T is the experimental incubation temperature (in K). Models were fit using the rTPC package of R57,58. Confidence intervals on parameter values were defined as the range ±2 standard errors from the model estimate; estimates were considered significantly different when these intervals did not overlap for different traits. We focus our discussion on the parameters e and Th, as these were deemed most biologically relevant to this system and could be accurately estimated for all traits given the range of temperatures for which data were available. Because we measured a single virus trait, we could not conduct formal statistical tests to compare parameter estimates between bees and viruses; however, we highlight instances where 95% confidence intervals were non-overlapping in virus and host. Confidence intervals on predicted rates were obtained by bootstrap resampling of the model residuals (10,000 model iterations) using R package “car”59. To display the temperature responsiveness of all traits on the same scale, we scaled each thermal performance curve to the trait’s maximum model-predicted value to yield a proportion of peak activity at each temperature.
Statistics and reproducibility
Numbers of replicates are described in the details of each experiment. All computed statistics are described in “Methods: Estimation of thermal performance curves”.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This project was supported by the USDA Agricultural Research Service Beltsville Bee Research Laboratory in house fund; USDA-NIFA Pollinator Health Grant 2020-67013-31861 to J.D.E.; and a North American Pollinator Protection Campaign Honey Bee Health Improvement Project Grant and an Eva Crane Trust Grant to E.C.P.Y. and J.D.E. Funders had no role in study design, data collection and interpretation, or publication. We thank the reviewers for their service in improving the manuscript.
Author contributions
E.C.P.Y., E.V.R., and J.D.E. conceived the study. All authors (E.C.P.Y., E.V.R., L.M.M., D.L.B., K.G., A.P., R.P., J.D.E.) conducted experiments. E.C.P.Y. analyzed data and drafted the manuscript. All authors (E.C.P.Y., E.V.R., L.M.M., D.L.B., K.G., A.P., R.P., J.D.E.) revised the manuscript and gave approval for publication.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Nicolas Desneux and Luke R. Grinham. Peer reviewer reports are available.
Data availability
All data are supplied in the Supplementary Information, Supplementary Data 1.
Competing interests
All authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-023-04704-6.
References
- 1.Molnár PK, Sckrabulis JP, Altman KA, Raffel TR. Thermal performance curves and the metabolic theory of ecology—a practical guide to models and experiments for parasitologists. J. Parasitol. 2017;103:423–439. doi: 10.1645/16-148. [DOI] [PubMed] [Google Scholar]
- 2.Rohr JR, et al. Frontiers in climate change–disease research. Trends Ecol. Evol. 2011;26:270–277. doi: 10.1016/j.tree.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dell AI, Pawar S, Savage VM. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl Acad. Sci. 2011;108:10591–10596. doi: 10.1073/pnas.1015178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirk D, et al. Empirical evidence that metabolic theory describes the temperature dependency of within-host parasite dynamics. PLOS Biol. 2018;16:e2004608. doi: 10.1371/journal.pbio.2004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohr JR, Raffel TR. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc. Natl Acad. Sci. 2010;107:8269–8274. doi: 10.1073/pnas.0912883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mordecai EA, et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 2019;22:1690–1708. doi: 10.1111/ele.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoolfield RM, Sharpe PJH, Magnuson CE. Non-linear regression of biological temperature-dependent rate models based on absolute reaction-rate theory. J. Theor. Biol. 1981;88:719–731. doi: 10.1016/0022-5193(81)90246-0. [DOI] [PubMed] [Google Scholar]
- 8.Dell AI, Pawar S, Savage VM. Temperature dependence of trophic interactions are driven by asymmetry of species responses and foraging strategy. J. Anim. Ecol. 2014;83:70–84. doi: 10.1111/1365-2656.12081. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JM, et al. The thermal mismatch hypothesis explains host susceptibility to an emerging infectious disease. Ecol. Lett. 2017;20:184–193. doi: 10.1111/ele.12720. [DOI] [PubMed] [Google Scholar]
- 10.Padfield D, Castledine M, Buckling A. Temperature-dependent changes to host–parasite interactions alter the thermal performance of a bacterial host. ISME J. 2020;14:389–398. doi: 10.1038/s41396-019-0526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stahlschmidt ZR, Adamo SA. Context dependency and generality of fever in insects. Naturwissenschaften. 2013;100:691–696. doi: 10.1007/s00114-013-1057-y. [DOI] [PubMed] [Google Scholar]
- 12.Heinrich, B. The hot-blooded insects: strategies and mechanisms of thermoregulation (Springer Science & Business Media, 2013).
- 13.Palmer-Young EC, et al. Temperature dependence of parasitic infection and gut bacterial communities in bumble bees. Environ. Microbiol. 2019;21:4706–4723. doi: 10.1111/1462-2920.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boorstein SM, Ewald PW. Costs and benefits of behavioral fever in Melanoplus sanguinipes infected by Nosema acridophagus. Physiol. Zool. 1987;60:586–595. doi: 10.1086/physzool.60.5.30156132. [DOI] [Google Scholar]
- 15.Hollings M. Disease control through virus-free stock. Annu. Rev. Phytopathol. 1965;3:367–396. doi: 10.1146/annurev.py.03.090165.002055. [DOI] [Google Scholar]
- 16.James RR. Temperature and chalkbrood development in the alfalfa leafcutting bee, Megachile rotundata. Apidologie. 2005;36:15–23. doi: 10.1051/apido:2004065. [DOI] [Google Scholar]
- 17.Boltaña S, et al. Behavioural fever is a synergic signal amplifying the innate immune response. Proc. R. Soc. B. 2013;280:20131381. doi: 10.1098/rspb.2013.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casadevall A. Thermal restriction as an antimicrobial function of fever. PLoS Pathog. 2016;12:e1005577. doi: 10.1371/journal.ppat.1005577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cevallos RC, Sarnow P. Temperature protects insect cells from infection by cricket paralysis virus. J. Virol. 2010;84:1652–1655. doi: 10.1128/JVI.01730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Himmer A. Ein Beitrag zur Kenntnis des Wärmehaushalts im Nestbau sozialer Hautflügler. Z. F.ür. Vgl. Physiol. 1927;5:375–389. doi: 10.1007/BF00338020. [DOI] [Google Scholar]
- 21.Esch H. Über die Körpertemperaturen und den Wärmehaushalt von Apis mellifica. Z. F.ür. Vgl. Physiol. 1960;43:305–335. doi: 10.1007/BF00298066. [DOI] [Google Scholar]
- 22.Heinrich B, Esch H. Thermoregulation in Bees. Am. Sci. 1994;82:164–170. [Google Scholar]
- 23.Fahrenholz L, Lamprecht I, Schricker B. Thermal investigations of a honey bee colony: thermoregulation of the hive during summer and winter and heat production of members of different bee castes. J. Comp. Physiol. B. 1989;159:551–560. doi: 10.1007/BF00694379. [DOI] [Google Scholar]
- 24.Harrison JF, Fewell JH. Environmental and genetic influences on flight metabolic rate in the honey bee, Apis mellifera. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2002;133:323–333. doi: 10.1016/S1095-6433(02)00163-0. [DOI] [PubMed] [Google Scholar]
- 25.Schmolz E, Hoffmeister D, Lamprecht I. Calorimetric investigations on metabolic rates and thermoregulation of sleeping honeybees (Apis mellifera carnica) Thermochim. Acta. 2002;382:221–227. doi: 10.1016/S0040-6031(01)00740-7. [DOI] [Google Scholar]
- 26.Groh C, Tautz J, Rössler W. Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc. Natl Acad. Sci. USA. 2004;101:4268–4273. doi: 10.1073/pnas.0400773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans JD, Schwarz RS. Bees brought to their knees: microbes affecting honey bee health. Trends Microbiol. 2011;19:614–620. doi: 10.1016/j.tim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Cornman RS, et al. Pathogen webs in collapsing honey bee colonies. PLOS ONE. 2012;7:e43562. doi: 10.1371/journal.pone.0043562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garedew A, Schmolz E, Lamprecht I. Microcalorimetric and respirometric investigation of the effect of temperature on the antivarroa action of the natural bee product-propolis. Thermochim. Acta. 2003;399:171–180. doi: 10.1016/S0040-6031(02)00453-7. [DOI] [Google Scholar]
- 30.Starks PT, Blackie CA, Seeley TD. Fever in honeybee colonies. Naturwissenschaften. 2000;87:229–231. doi: 10.1007/s001140050709. [DOI] [PubMed] [Google Scholar]
- 31.Martín-Hernández R, et al. Effect of temperature on the biotic potential of honeybee microsporidia. Appl. Environ. Microbiol. 2009;75:2554–2557. doi: 10.1128/AEM.02908-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Prisco G, et al. Dynamics of persistent and acute deformed wing virus infections in honey bees, Apis mellifera. Viruses. 2011;3:2425–2441. doi: 10.3390/v3122425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalmon A, Peruzzi M, Le Conte Y, Alaux C, Pioz M. Temperature-driven changes in viral loads in the honey bee Apis mellifera. J. Invertebr. Pathol. 2019;160:87–94. doi: 10.1016/j.jip.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Bailey L. Recent research on honeybee viruses. Bee World. 1975;56:55–64. doi: 10.1080/0005772X.1975.11097544. [DOI] [Google Scholar]
- 35.de Miranda JR, Genersch E. Deformed wing virus. J. Invertebr. Pathol. 2010;103:S48–S61. doi: 10.1016/j.jip.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Martin SJ, Brettell LE. Deformed wing virus in honeybees and other insects. Annu. Rev. Virol. 2019;6:49–69. doi: 10.1146/annurev-virology-092818-015700. [DOI] [PubMed] [Google Scholar]
- 37.Manley R, Boots M, Wilfert L. REVIEW: emerging viral disease risk to pollinating insects: ecological, evolutionary and anthropogenic factors. J. Appl. Ecol. 2015;52:331–340. doi: 10.1111/1365-2664.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tehel A, Brown MJ, Paxton RJ. Impact of managed honey bee viruses on wild bees. Curr. Opin. Virol. 2016;19:16–22. doi: 10.1016/j.coviro.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 39.McMenamin AJ, Parekh F, Lawrence V, Flenniken ML. Investigating virus–host interactions in cultured primary honey bee cells. Insects. 2021;12:653. doi: 10.3390/insects12070653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraus B, Velthuis HHW, Tingek S. Temperature profiles of the brood nests of Apis cerana and Apis mellifera colonies and their relation to varroosis. J. Apic. Res. 1998;37:175–181. doi: 10.1080/00218839.1998.11100969. [DOI] [Google Scholar]
- 41.Levin CG, Collison CH. Broodnest temperature differences and their possible effect on drone brood production and distribution in honeybee colonies. J. Apic. Res. 1990;29:35–44. doi: 10.1080/00218839.1990.11101195. [DOI] [Google Scholar]
- 42.Evans JD, Banmeke O, Palmer-Young EC, Chen Y, Ryabov EV. Beeporter: tools for high-throughput analyses of pollinator-virus infections. Mol. Ecol. Resour. 2022;22:978–987. doi: 10.1111/1755-0998.13526. [DOI] [PubMed] [Google Scholar]
- 43.Yuan X, Kadowaki T. DWV 3C protease uncovers the diverse catalytic triad in insect RNA viruses. Microbiol. Spectr. 2022;10:e00068–22. doi: 10.1128/spectrum.00068-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- 45.Petz M, Stabentheiner A, Crailsheim K. Respiration of individual honeybee larvae in relation to age and ambient temperature. J. Comp. Physiol. B. 2004;174:511–518. doi: 10.1007/s00360-004-0439-z. [DOI] [PubMed] [Google Scholar]
- 46.Becher MA, Scharpenberg H, Moritz RFA. Pupal developmental temperature and behavioral specialization of honeybee workers (Apis mellifera L.) J. Comp. Physiol. A. 2009;195:673–679. doi: 10.1007/s00359-009-0442-7. [DOI] [PubMed] [Google Scholar]
- 47.Allen MD. Respiration rates of worker honeybees of different ages and at different temperatures. J. Exp. Biol. 1959;36:92–101. doi: 10.1242/jeb.36.1.92. [DOI] [Google Scholar]
- 48.Doublet V, Natsopoulou ME, Zschiesche L, Paxton RJ. Within-host competition among the honey bees pathogens Nosema ceranae and Deformed wing virus is asymmetric and to the disadvantage of the virus. J. Invertebr. Pathol. 2015;124:31–34. doi: 10.1016/j.jip.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Valles SM, Porter SD. Influence of temperature on the pathogenicity of Solenopsis invicta virus 3. J. Invertebr. Pathol. 2019;166:107217. doi: 10.1016/j.jip.2019.107217. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi M, Inagaki S, Kawase S. Effect of high temperature on the development of nuclear polyhedrosis virus in the silkworm, Bombyx mori. J. Invertebr. Pathol. 1981;38:386–394. doi: 10.1016/0022-2011(81)90106-3. [DOI] [Google Scholar]
- 51.de Roode JC, Lefèvre T, Hunter MD, Lefevre T, Hunter MD. Self-medication in animals. Science. 2013;340:150–151. doi: 10.1126/science.1235824. [DOI] [PubMed] [Google Scholar]
- 52.Campbell J, Kessler B, Mayack C, Naug D. Behavioural fever in infected honeybees: parasitic manipulation or coincidental benefit? Parasitology. 2010;137:1487–1491. doi: 10.1017/S0031182010000235. [DOI] [PubMed] [Google Scholar]
- 53.Fürst MA, McMahon DP, Osborne JL, Paxton RJ, Brown MJF. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature. 2014;506:364–366. doi: 10.1038/nature12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryabov EV, et al. Development of a honey bee RNA virus vector based on the genome of a deformed wing virus. Viruses. 2020;12:374. doi: 10.3390/v12040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kovac H, Stabentheiner A, Hetz SK, Petz M, Crailsheim K. Respiration of resting honeybees. J. Insect Physiol. 2007;53:1250–1261. doi: 10.1016/j.jinsphys.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Padfield D, Yvon‐Durocher G, Buckling A, Jennings S, Yvon‐Durocher G. Rapid evolution of metabolic traits explains thermal adaptation in phytoplankton. Ecol. Lett. 2016;19:133–142. doi: 10.1111/ele.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Padfield D, O’Sullivan H, Pawar S. rTPC and nls.multstart: a new pipeline to fit thermal performance curves in r. Methods Ecol. Evol. 2021;12:1138–1143. doi: 10.1111/2041-210X.13585. [DOI] [Google Scholar]
- 58.R Core Team. R: a language and environment for statistical computing (R Foundation for Statistical Computing, 2014).
- 59.Fox, J. & Weisberg, S. An R companion to applied regression (Sage, 2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data are supplied in the Supplementary Information, Supplementary Data 1.