Abstract
Introduction
Progressive cerebral venous sinus thrombosis (CVST)-induced visual loss remains problematic, despite decreasing overall mortality owing to early diagnosis and aggressive treatment. Optic nerve sheath fenestration (ONSF) improves or stabilizes visual function in patients with idiopathic intracranial hypertension; however, its role in CVST awaits elucidation. We evaluated the efficacy and safety of ONSF in resolving CVST-induced visual impairment based on long-term observation.
Methods
This observational study included 18 patients with progressive CVST-induced visual loss, who had undergone ONSF between 2012 and 2021. Patients received maximum medical therapy, including anticoagulants and intracranial pressure (ICP)-lowering medications. The best-corrected visual acuity (BCVA), visual fields (VFs), and optic nerve head were assessed at baseline, at 1 week after ONSF, and over 6 months after ONSF. Activities of daily living (ADL) and National Eye Institute Visual Function Questionnaire-25 (VFQ-25) scores were assessed at final follow-up.
Results
Thirty-one ONSF-treated eyes of 18 patients were included. The mean follow-up duration was 35.6 months (range 1 week–8 years). Two patients were lost to follow-up. Before ONSF, all patients were still experiencing progressive visual loss despite receiving adequate anticoagulation and ICP-lowering therapy. Postoperative BCVA remained stable or improved in 25/31 eyes (80.6%) 1 week postoperatively and 17/28 eyes (60.7%) upon final follow-up. All papilledema resolved postoperatively. No complications were reported except for one transient postoperative diplopia. The median ADL score was 100 (range 25–100), and the mean total VFQ-25 score was 40.6 (range 9.5–87.3).
Conclusion
This was the largest study to describe ONSF’s role in CVST based on a long-term follow-up. Considering its efficacy and favorable safety, ONSF can be considered an important adjunctive approach to resolving progressive visual loss of CVST patients, on the basis of anticoagulation and ICP-lowering therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-022-00434-9.
Keywords: Cerebral venous sinus thrombosis, Optic nerve sheath fenestration, Visual loss, Papilledema, Intracranial hypertension
Plain Language Summary
Cerebral venous sinus thrombosis (CVST) is a cerebrovascular disease that generally affects young patients. Medical treatments include anticoagulants, intracranial pressure (ICP)-lowering medications, and repeated lumbar punctures, effectively reducing CVST’s mortality rate. However, CVST still carries a potential risk of progressive vision loss. Optic nerve sheath fenestration (ONSF) has been reported to be effective and safe in protecting visual function of patients with idiopathic intracranial hypertension. However, its efficacy and safety have not been evaluated in visual loss caused by CVST. We were the first to evaluate the efficacy and safety of ONSF in CVST-induced progressive visual loss based on long-term follow-ups. Before ONSF, all patients were still experiencing progressive visual loss despite receiving adequate anticoagulation and ICP-lowering therapy. We found ONSF to be 80.6% (1 week postoperatively) and 60.7% (after long-term follow-up of over 6 months) effective in stabilizing and/or improving visual function as well as 100% effective in papilledema resolution. Moreover, ONSF exhibited a favorable safety profile, with an extremely low complication rate of 5.6% despite under perioperative anticoagulation. Although visual impairment in CVST was reported to be uncommon, it often significantly affects quality of life and social value of patients. Thus, visual loss in CVST deserves more attention from neurologists, neurosurgeons, and ophthalmologists. Considering its efficacy and favorable safety, ONSF could be regarded a potentially important adjunctive approach to resolving progressive visual loss in CVST patients, on the basis of anticoagulation and ICP-lowering therapy.
Procedural videos available for this article.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-022-00434-9.
Key Summary Points
| Why carry out this study? |
| Cerebral venous sinus thrombosis (CVST), a cerebrovascular disease characterized by occlusion of cerebral veins or sinuses and the subsequent development of intracranial hypertension, can lead to progressive visual loss and even complete blindness. |
| Optic nerve sheath fenestration (ONSF) has been reported to be effective and safe in protecting visual function of patients with idiopathic intracranial hypertension, but its efficacy and safety have not been evaluated in visual loss secondary to CVST. |
| This study is the first to evaluate the efficacy and safety of ONSF in visual loss secondary to CVST based on long-term follow-ups. |
| What was learned from this study? |
| In this study, before ONSF, all patients were still experiencing progressive visual loss despite receiving adequate anticoagulation and ICP-lowering therapy. We found ONSF to be 80.6% (1 week postoperatively) and 60.7% (over 6 months postoperatively) effective in stabilizing and/or improving visual function of CVST, as well as 100% effective in papilledema resolution; moreover, it exhibited a favorable safety profile despite under perioperative anticoagulation, with an extremely low complication rate of 5.6%. |
| This study shows that progressive visual loss remains a big challenge in CVST, which deserves more attention from neurologists, ophthalmologists, and neurosurgeons. |
| Considering its efficacy and favorable safety, ONSF can be regarded a potentially important adjunctive approach to resolving CVST-induced progressive visual loss, on the basis of anticoagulation and ICP-lowering therapy. |
Digital Features
This article is published with digital features, including procedural videos, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.21723761.
Introduction
Cerebral venous sinus thrombosis (CVST) is a cerebrovascular disease characterized by occlusion of cerebral veins or sinuses and the subsequent development of intracranial hypertension (IH), owing to impaired cerebrospinal fluid (CSF) absorption [1]. Medical treatments of CVST include anticoagulants, intracranial pressure (ICP)-lowering medications, and repeated lumbar punctures, effectively reducing CVST’s mortality rate [2]. Nevertheless, CVST carries a potential risk of progressive vision loss. Despite receiving maximum medical therapy, 6.7–40% of patients are estimated to suffer from visual impairment and even progress to complete blindness [3–6], especially those with diagnostic delay or chronic IH, at least partly due to prolonged optic nerve compression [7, 8]. Furthermore, since CVST is more common among young and middle-aged people [9], visual impairment often significantly impacts family and socioeconomic development. Therefore, it is critical for neurologists, ophthalmologists, and neurosurgeons to consider surgery when visual loss progresses, despite maximum medical therapy. Optic nerve sheath fenestration (ONSF), which involves cutting slits or windows in the optic nerve sheath to allow CSF to escape, thus relieving optic nerve compression, has been shown to be effective and safe in protecting visual function of patients with idiopathic intracranial hypertension (IIH) [10–13]. However, the mechanism of visual loss in CVST including primary venous ischemia and secondary intracranial hypertension is more complicated than that in IIH. It is unclear whether ONSF could help improve visual impairment in CVST. Concerns about surgical risks of intraoperative bleeding from anticoagulation greatly limit the use of ONSF in CVST, as most patients have been receiving long-term anticoagulation therapy since diagnosis, so few sporadic cases with relatively short-term follow-ups describing ONSF utility in CVST have been reported [3, 14, 15]. Therefore, an evaluation of ONSF’s efficacy and safety for CVST based on long-term observations is of paramount important, while anticoagulants are used simultaneously. Our purpose is to evaluate the efficacy and safety of ONSF in resolving CVST-induced visual impairment based on long-term observation. Here, we present our long-term follow-up results of ONSF in a population of CVST-induced progressive visual loss that is larger than previously studied.
Methods
Study Design and Patients
The medical records of 18 CVST patients with IH who underwent ONSF between March 2012 and November 2021 were retrospectively reviewed. These patients were referred from the neurology departments of hospitals to our ophthalmology department for progressive visual loss despite adequate medical treatment. CVST was diagnosed by neurologists based on clinical manifestations and magnetic resonance imaging/venography (MRI/MRV) findings. The inclusion criteria were as follows: (1) diagnosed with CVST; (2) raised ICP (CSF opening pressure > 250 mm H2O) detected by lumber puncture; (3) progressive visual loss as evidenced by deteriorating visual acuity or visual fields (VFs) during preoperative evaluation (a decrease of at least two lines on the Early Treatment Diabetic Retinopathy Study (ETDRS) chart or at least one level on the scale for visual acuity, or a decrease of at least 5 dB for VFs) in spite of treatment with adequate anticoagulants and/or ICP-lowering drugs; (4) no history of other ocular disorders or ocular surgery; (5) had undergone ONSF surgery; and (6) had at least one postoperative follow-up. The exclusion criteria included having a history of other ocular disorders or ocular surgery or refusal to complete postoperative follow-ups. This study was reviewed and approved by the Medical Ethical Committee of the Shanghai General Hospital (2020-N–145). Each patient provided written informed consent prior to be included in the study. The study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Data Collection and Ophthalmological Examinations
Data included patient demographics, duration of visual loss and other symptoms, causes or risk factors, thrombosis sites, ocular and non-ocular manifestations, medical or surgical treatment, preoperative ICP, ophthalmological assessments, clinical outcomes, and complications.
Ophthalmological assessments included best-corrected visual acuity (BCVA), VFs, and fundoscopy. BCVA was measured using the ETDRS chart. For patients with BCVA worse than 20/800, “counting fingers” (CF), “hand movement” (HM), “light perception” (LP), and “no light perception” (NLP) were employed. VFs were assessed using automated perimetry (Humphrey Field Analyzer II; Carl Zeiss Meditec, Dublin, CA, USA). Visual field loss was measured as the mean deviation in decibels (dB), and more-negative scores indicated worse visual field loss. The optic nerve head was evaluated using an ophthalmoscope, and cross-sectional images of the optic nerve head were obtained using optical coherence tomography (Humphrey Field Analyzer II; Carl Zeiss Meditec).
Perioperative Management of Medications
A perioperative bridging anticoagulation strategy was administered to patients using anticoagulants based on pharmaco-metabolism. In patients using vitamin K antagonists such as warfarin preoperatively, warfarin was discontinued several days before ONSF, the international normalized ratio (INR) was monitored, and treatment was bridged with low-molecular-weight heparin (LMWH) until the INR was ≤ 1.6. In patients who used non-vitamin K oral anticoagulants (NOACs), such as rivaroxaban and dabigatran preoperatively, the NOACs were omitted 48 h before operation and subsequently bridged with LMWH. LMWH was suspended 12 h before ONSF. Anticoagulants were reinitiated 8 h after surgery. Intravenous steroids and antibiotics were administered for three consecutive days postoperatively.
Surgical Procedure
Indication for ONSF in all CVST cases was based on an increased ICP combined with a progressive visual loss despite maximal anticoagulants and ICP-lowering medications. All ONSFs were performed by the same surgeon (H.C.). Surgery was performed using a medial transconjunctival approach under general anesthesia, according to Farris [11].
Follow-up Measures
Short-term (1 week postoperatively) and long-term (more than 6 months postoperatively) follow-ups were conducted to assess ONSF’s efficacy and safety. At the first follow-up, patients underwent ophthalmological assessment at the hospital where ONSF was performed. At the final follow-up, patients were referred and transferred to local hospitals to assess visual function and fundus scope, predominantly via the internet or telephone.
BCVA improvement was defined as an improvement of at least two lines on the ETDRS chart or at least one level on the scale, such as from “NLP” to “LP” in patients with poor vision. VFs improvement was defined as an improvement of ≥ 5 dB. BCVA decline was defined as a decrease of at least two lines on the ETDRS chart or at least one level on the scale. A decline in VFs was defined as a decrease of at least 5 dB. Otherwise, it was defined as the maintenance of BCVA or VFs. Eyes remaining in the “NLP” state were calculated separately.
To determine the efficacy of ONSF in stabilizing and/or improving visual function, ONSF-received eyes were divided into “Effectiveness” and “Ineffectiveness” groups based on BCVA. “Effectiveness” was defined as an improvement or maintenance of BCVA, and “Ineffectiveness” was defined as a worsening BCVA or maintenance of the “NLP” status.
In addition, to assess generic health-related and vision-related quality of life (QoL), the activities of daily living (ADL) questionnaire and National Eye Institute Visual Function Questionnaire-25 (VFQ-25) were administered to patients or their families online at the final follow-up. The ADL evaluates a patient’s ability regarding feeding, bathing, grooming, dressing, bowel/bladder management, toilet use, transfers, mobility, and the use of stairs using the Barthel Index (BI). The BI is scored from 0 to 100, with a higher score indicating a higher degree of independence in daily life. A BI score ≥ 60 reflects basic self-care ability, and < 60 indicates functional dependency [16]. The VFQ-25 comprises 25 items related to vision-targeted QoL grouped into 12 subscales. The items in each subscale were averaged to obtain the 12 subscale scores. The overall VFQ-25 score was calculated using the average scores of all subscales, except for the general health domain [17].
Results
A total of 31 eyes from 18 patients who had undergone ONSF were included in the study. Their demographic and clinical characteristics are summarized in Table 1. Of the 18 patients, 9 were women (50%) and 9 were men (50%). Their mean age was 34 (range 8–53) years for women and 44 (range 4–69) years for men. The follow-up durations ranged from 1 week to 8 years, with a mean of 35.6 months. All patients underwent initial postoperative assessments 1 week after ONSF. Subsequently, except for two patients who were lost to follow-up (nos. 1 and 2), 16 patients underwent long-term follow-up for at least 6 months, with a mean of 40 months (range 6–96 months). The median duration of visual loss was 3.5 months (range 0.5–13 months). Other symptoms included headache, tinnitus, and diplopia. The median duration from onset to ONSF was 6.5 months (range 2–36 months).
Table 1.
Patient demographics and clinical characteristics
| No./age/sex | Time | No. of hospitalizations pre-ONSF | ICP (mmH2O) | Causes or risk factors | Locations of thrombosis | Other symptoms | Other medical conditions | CDT pre-ONSF | Medical treatment pre-ONSF | Follow-up time | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset to ONSF | Visual loss to ONSF | ||||||||||
| 1/34/F | 8 m | 3 m | 3 | 300 | Pregnancy, induced abortion | Straight sinus, right sigmoid sinus | – | – | – | Warfarin, mannitol | 1 w |
| 2/34/F | 6 m | 2.5 m | 4 | > 330a | Acoustic neuroma surgery | Right transverse sinus, right sigmoid sinus | Diplopia | – | – | LMWH, mannitol | 1 w |
| 3/30/M | 3 y | 20 d | 2 | 346 | Polycythemia | Superior sagittal sinus, inferior sagittal sinus, straight sinus, right sigmoid sinus, bilateral transverse sinuses | Headache, ophthalmalgia | Hypertension, hyperlipidemia, hypoalbuminemia | – | Warfarin, mannitol | 72 m |
| 4/42/M | 8 m | 15 d | 2 | > 330a | Unknown | Superior sagittal sinus | Headache, tinnitus | – | + | Warfarin, mannitol | 96 m |
| 5/4/M | 6 m | 6 m | 3 | 210 | Otitis media, mastoiditis, sinusitis | Cavernous sinus | Headache, tinnitus, dizziness, focal neurological deficits | – | + | Mannitol | 44 m |
| 6/35/M | 7 m | 4 m | 4 | > 330a | Unknown | Superior sagittal sinus, right transverse sinus, torcular herophili, bilateral sigmoid sinuses | Headache | – | – | LMWH, mannitol | 16 m |
| 7/50/M | 5 m | 5 m | 4 | > 330a | Unknown | Superior sagittal sinus, right transverse sinus, straight sinus | Dizziness, seizure | HF, DILI, HE, epilepsy, UTI, Hypoalbuminemia | + | LMWH, warfarin, acetazolamide | 60 m |
| 8/53/F | 2 m | 2 m | 4 | > 330a | Microvascular decompression for hemifacial spasm | Right transverse sinus | Headache, nausea and vomiting, metamorphopsia | T2DM, hypertension | – | LMWH, warfarin, acetazolamide | 51 m |
| 9/31/F | 7 m | 7 m | 3 | > 330a | Thrombocytosis, otitis media | Right transverse sinus, right sigmoid sinus, right internal jugular vein | Headache | – | – | Warfarin, mannitol | 38 m |
| 10/39/F | 13 m | 2 m | 3 | > 330a | Unknown | Superior sagittal sinus, bilateral transverse sinuses | Headache, tinnitus | – | – | Warfarin, mannitol | 58 m |
| 11/69/M | 13 m | 13 m | 3 | 310 | Non-Hodgkin lymphoma | Right sigmoid sinus | Headache | Posterior fossa dAVFs | – | Warfarin, mannitol | 38 m |
| 12/23/F | 5 m | 5 m | 2 | 360 | Unknown | Inferior sagittal sinus | Headache | – | + | Warfarin, mannitol | 80 m |
| 13/36/M | 6 m | 5 m | 3 | > 330a | Thrombocytosis | Left transverse sinus, left sigmoid sinus | Headache, dizziness, diplopia | – | – | LMWH, warfarin, acetazolamide | 17 m |
| 14/29/F | 3 m | 3 m | 3 | > 330a | Unknown | Left transverse sinus, left sigmoid sinus | – | PA, adrenocortical adenoma, secondary hypertension | – | Mannitol | 40 m |
| 15/32/F | 11 m | 11 m | 2 | > 330a | Thrombocytosis,induced abortion | Superior sagittal sinus, torcular herophili, | Headache, tinnitus, amaurosis fugax | – | – | LMWH, mannitol | 7 m |
| 16/8/F | 13 m | 7 m | 3 | > 330a | S protein deficiency | Bilateral transverse sinuses, bilateral sigmoid sinuses, torcular herophili, | Headache, tinnitus, vomiting | – | + | Warfarin, mannitol | 11 m |
| 17/49/M | 2 m | 2 m | 2 | > 330a | Short-term plateau living | Superior sagittal sinus, left transverse sinus, left sigmoid sinus, torcular herophili, left internal jugular vein | Headache, dizziness, diplopia, vomiting | Hyperlipidemia | + | Dabigatran, acetazolamide | 6 m |
| 18/69/M | 6 m | 3 m | 2 | > 330a | Craniocerebral trauma | Superior sagittal sinus, right transverse sinus, right sigmoid sinus | Headache, amaurosis fugax | Ischemic stroke, T2DM, AF | + | LMWH, rivaroxaban, mannitol | 6 m |
AF atrial fibrillation, CDT catheter directed thrombolysis, dAVFs dural arteriovenous fistulas, DILI drug-induced liver injury, HE hepatic encephalopathy, HF hepatic failure, ICP intracranial pressure, LMWH Low-molecular-weight heparin, ONSF optic nerve sheath fenestration, PA primary aldosteronism, T2DM type 2 diabetes mellitus, UTI urinary tract infection, d day, m month, w week, y year
+ yes, - no or none
aIn cases with ICP over 330 mmH2O the exact ICP values were not recorded
All patients were treated with ICP-lowering medications such as acetazolamide and mannitol; 16 were treated with anticoagulants, and 2 had discontinued oral anticoagulants for a long time. Of the patients, 7 (38.9%) underwent catheter-directed thrombolysis before ONSF. Causes or risk factors were identified in 12 patients, and 6 had no identifiable cause, despite extensive laboratory examination. The predominant cause of CVST was the patient’s underlying disease, which induced various hypercoagulable states
MRI/MRV imaging revealed that the following vessels were involved, in descending order: the transverse sinus, sigmoid sinus, superior sagittal sinus, torcular herophili, straight sinus, inferior sagittal sinus, internal jugular vein, and cavernous sinus. Moreover, 13 (72.2%) patients had multiple sinus thromboses, and 5 (27.8%) had single sinus thrombosis.
Visual Function Before and After Optic Nerve Sheath Fenestration
Before ONSF, all patients were still experiencing progressive visual loss despite receiving adequate anticoagulation and ICP-lowering therapy in neurology departments, and physicians often had no better treatment for the visual loss.
Among 18 patients included, 13 underwent bilateral ONSF, of which 4 initially underwent unilateral ONSF in the eye with worse visual acuity, followed by a second ONSF in the other eye 1 week later. The remaining 5 patients only received unilateral ONSF in the worse eye because they had no significant visual acuity loss in the other eye. The BCVA, VFs, and ophthalmoscopy findings before and after ONSF surgery are described in Table 2 and Fig. 1.
Table 2.
Ocular parameters pre- and post-optic nerve sheath fenestration
| NO | Eyes | ONSF | Papilledema pre-/post-ONSF | BCVA | VF (MD, db) | Effectivenessa | Complication | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-ONSF | 1w post-ONSF | Last visit | Pre-ONSF | Post-ONSF | 1w post-ONSF | Last visit | ||||||
| 1 | R | − | ± | 20/20 | 20/20 | N/A | − 3.81 | − 2.77 | / | / | − | |
| L | + | ± | 20/32 | 20/40 | N/A | − 8.70 | − 7.15 | + | N/A | |||
| 2 | R | + | ± | CF | 20/200 | N/A | N/A | N/A | + | N/A | − | |
| L | + | ± | CF | 20/50 | N/A | N/A | N/A | + | N/A | |||
| 3a | R | + | ± | CF | 20/500 | CF | − 35.96 | − 31.61 | + | + | − | |
| L | + | ± | LP | 20/500 | CF | N/A | − 34.74 | + | + | |||
| 4a | R | + | ± | 20/640 | 20/250 | LP | − 26.18 | N/A | + | − | − | |
| L | + | ± | 20/63 | 20/100 | CF | − 16.45 | N/A | − | − | |||
| 5 | R | − | ± | 20/63 | 20/63 | 20/25 | N/A | − 2.40 | / | / | − | |
| L | + | ± | 20/125 | 20/125 | 20/40 | N/A | − 22.28 | + | + | |||
| 6 | R | + | ± | NLP | NLP | NLP | N/A | N/A | − | − | − | |
| L | + | ± | HM | HM | HM | N/A | N/A | + | + | |||
| 7 | R | + | ± | NLP | LP | NLP | N/A | N/A | + | − | − | |
| L | + | ± | NLP | LP | NLP | N/A | N/A | + | − | |||
| 8 | R | + | ± | HM | HM | NLP | N/A | N/A | + | − | − | |
| L | − | ± | 20/125 | 20/125 | NLP | N/A | N/A | / | / | |||
| 9 | R | + | OA/OA | NLP | NLP | NLP | N/A | N/A | − | − | − | |
| L | + | OA/OA | NLP | NLP | NLP | N/A | N/A | − | − | |||
| 10 | R | + | OA/OA | CF | CF | CF | N/A | N/A | + | + | − | |
| L | − | ± | 20/25 | 20/20 | 20/25 | − 14.50 | − 24.00 | / | / | |||
| 11a | R | + | OA/OA | 20/63 | 20/63 | 20/200 | − 27.88 | N/A | + | − | − | |
| L | + | OA/OA | 20/32 | 20/40 | 20/100 | − 26.62 | N/A | + | − | |||
| 12a | R | + | ± | CF | 20/400 | 20/100 | − 28.96 | − 25.55 | + | + | − | |
| L | + | ± | 20/80 | 20/80 | 20/80 | − 28.01 | − 24.01 | + | + | |||
| 13 | R | + | ± | 20/100 | 20/100 | 20/63 | − 31.09 | N/A | + | + | − | |
| L | + | ± | NLP | NLP | NLP | N/A | N/A | − | − | |||
| 14 | R | + | ± | 20/200 | 20/200 | 20/160 | − 30.65 | − 28.82 | + | + | − | |
| L | + | ± | 20/400 | 20/400 | 20/400 | − 31.30 | − 28.56 | + | + | |||
| 15 | R | + | ± | 20/50 | 20/40 | 20/40 | − 32.12 | − 29.95 | + | + | − | |
| L | + | ± | NLP | HM | HM | N/A | − 31.63 | + | + | |||
| 16 | R | + | OA/OA | NLP | CF | 20/200 | N/A | N/A | + | + | − | |
| L | + | OA/OA | 20/200 | 20/160 | 20/100 | N/A | N/A | + | + | |||
| 17 | R | − | ± | 20/20 | 20/20 | 20/20 | 0.06 | 1.30 | / | / | Transient diplopia | |
| L | + | ± | 20/25 | 20/20 | 20/20 | − 0.88 | 1.30 | + | + | |||
| 18 | R | + | ± | 20/32 | 20/40 | 20/25 | − 22.31 | − 12.08 | + | + | − | |
| L | + | ± | 20/32 | 20/50 | 20/25 | − 7.16 | − 14.64 | − | + | |||
L left, R right, CF counting fingers, HM hand movement, LP light perception, NLP no light perception, N/A not available, OA optic atrophy, ONSF optic nerve sheath fenestration
+ yes, − no or none
aONSF was performed in the eye with worse visual acuity first and then the other eye 1 week later
Fig. 1.
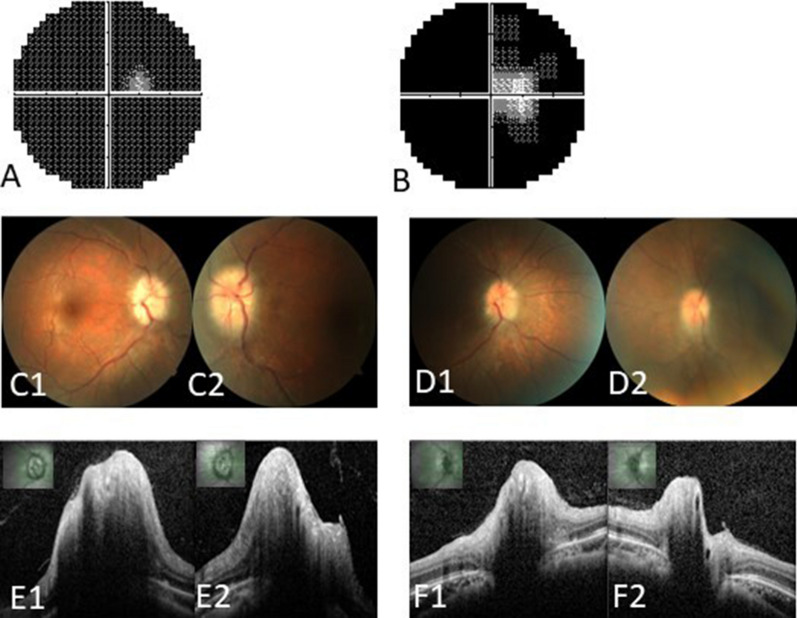
The preoperative and postoperative visual findings of a representative case. A 32-year-old female (no. 15) presented with progressive visual loss for 11 months, accompanied by headaches, amaurosis fugax, and tinnitus. She was diagnosed with CVST secondary to thrombocytosis and treated with intravenous heparin and mannitol for 1 month, but her vision loss further progressed. On admission, the BCVA was 20/50 in the right eye and “NLP” in the left, and the right visual field exhibited a tubular aspect (A). Fundus examination revealed papilledema and retinal vein tortuosity in both eyes (C1, C2). Optic disc optic coherence tomography showed bilateral significant papilledema and increased RNFL thickness (E1, E2). Four days after optic nerve sheath fenestration, the BCVA improved to 20/40 in the right and “HM” in the left, and right visual-field defects also improved (B), papilledema noticeably resolved (D1, D2), and bilateral RNFL thickness was decreased (F1, F2). BCVA best-corrected visual acuity, HM hand movement, NLP no light perception, RNFL retinal nerve fiber layer
At the 1-week postoperative follow-up, BCVA improved in 10 (32.3%) eyes, remained stable in 15 (48.4%), worsened in 2 (6.5%), and remained in the “NLP” state in 4 (12.9%). Notably, among 8 eyes with an “NLP” status before ONSF, 4 improved to “LP,” “HM,” or “CF” status (patient NO. 7, 15, 16) in the first postoperative week, although another 4 were still in NLP.
On assessing the 28 eyes at the final follow-up, 7 (25.0%) exhibited improvement, 10 (35.7%) maintained their original state, 5 (17.9%) worsened, and 6 (21.4%) remained in the “NLP” state compared with preoperative BCVA.
Due to poor visual acuity or lack of compliance, 11 patients failed to undergo preoperative and postoperative VFs examinations. Finally, VFs from 10 eyes of 7 patients were available both preoperatively and postoperatively. VFs improved in 1 (10%) eye, remained stable in 8 (80%), and worsened in 1 (10%).
On performing ophthalmoscopy on the 31 eyes that had undergone ONSF, papilledema was detected in 24 (77.4%) eyes preoperatively, and optic disk atrophy was detected in 7 (22.6%) eyes. All papilledema were resolved after ONSF.
Based on our definition of "Effectiveness” and “Ineffectiveness” in methods, at the 1-week postoperative follow-up, 25 of 31 (80.6%) eyes demonstrated “Effectiveness,” while 6 of 31 eyes (19.4%) exhibited “Ineffectiveness.” At the final visit, 17 of 28 eyes (60.7%) demonstrated “Effectiveness,” while 11 of 28 eyes (39.3%) exhibited “Ineffectiveness.”
The Safety of Optic Nerve Sheath Fenestration
No vision-threatening intraoperative or postoperative complications were observed, except for one patient who experienced transient diplopia (Table 2). Our study did not observe other commonly reported complications, such as atonic pupil and motility disorders (Table 3). Despite adequate perioperative anticoagulation, no significant intra-operative hemorrhage was observed, except for mild oozing, which did not affect the surgery performance, as shown in Videos 1 and 2.
Table 3.
Representative studies concerning complications of optic nerve sheath fenestration in idiopathic intracranial hypertension and others
| Studies | Numbers | Diagnosis | Overall complications | Intra-operative complications | Motility disorders/diplopia | Pupillary dysfunction | Visual loss due to ONSF | Other |
|---|---|---|---|---|---|---|---|---|
|
Plotnik and Kosmorsky [27]a |
31 (38 eyes) | IIH, NAION, and others | 44.7% | 5.3% | 28.9% (100%b) | 10.5% (75%b) | 10.5% (25%b) | 0% |
| Banta and Farris [10] | 86 | IIH | 45.3% | 0% | 34.9% (87%b) | 7% | 2.4% (50%b) | 7.0% (Corneal dellen) |
| Chandrasekaran et al. [12] | 32 | IIH and others | 15.6% | 0% | 9.4% (100%b) | 6.3% (100%b) | 0% | 3.1% (Disc hemorrhage, 100%b) |
| Moreau et al. [11] | 331 | IIH, NAION, OND, and others | 6.9% | 0% | 6.0% (60%b) | 0% | 0% | 1.1% (Corneal dellen or conjunctival pyogenic granuloma, 100%b) |
| Obi et al. [13] | 14 | IIH | 9.7% | 0% | 6.5% (100%b) | 0% | 0% | 3.2% (Ocular discomfort, 100%b) |
| Vaidya et al. [28] | 104 | IIH | 23.0% | 0% | 6.7% (100%b) | 16.3% | 0% | 0% |
| Bersani et al. [29] | 42 | IIH | 2.6% | 0% | 2.6% (0%b) | 0% | 0% | 0% |
IIH idiopathic intracranial hypertension, NAION non-arteritic ischemic optic neuropathy, OND optic nerve drusen, ONSF optic nerve sheath fenestration
a In this study, the complication rates were measured based on the number of eyes
b The percentage of transient complications among total complications was shown in the brackets
Supplementary file2 Video 1. Optic nerve sheath fenestration was performed on an idiopathic intracranial hypertension patient. No intraoperative complication was observed. (MP4 21219 KB)
Supplementary file3 Video 2. Optic nerve sheath fenestration was performed on a CVST patient (no. 15) who had been treated with intravenous heparin and mannitol for 1 month. The perioperative anticoagulation and bridging strategy mentioned in the manuscript was used. Compared with the surgery performed in IIH (Video 1), it showed only mild oozing, which could be easily stopped by cotton pieces and did not affect the performance of the surgery. (MP4 22080 KB)
Quality of Life-Questionnaire Results After Optic Nerve Sheath Fenestration
At the final follow-up, the average BI score for ADL was 91 (range 25–100), with a median score of 100. Furthermore, 77.8% (n = 14) of the patients maintained basic self-care ability in life (BI > 60). However, the mean total VFQ-25 score was 40.6 (range 9.5–87.3), indicating a more severe impact of visual loss on patients’ vision-related QoL than on health-related QoL. The 12 subscale scores in the VFQ-25 ranged from 21.7 (“dependency” subscale) to 90 (“ocular pain” subscale). “Dependency,” “driving,” and “general vision” subscales yielded the lowest scores. Furthermore, all patients exhibited varying degrees of impairment across the four subgroups, “general health,” “mental health,” “role difficulties,” and “dependency.” ADL and VFQ-25 results at the final visit are shown in Table S1.
Discussion
This is the largest observational study involving long-term follow-up to assess the efficacy and safety of ONSF in CVST. ONSF was 80.6% (1 week postoperatively) and 60.7% (after long-term follow-up) effective in stabilizing and/or improving visual function, as well as 100% effective in papilledema resolution. Moreover, it exhibited a favorable safety profile with an extremely low complication rate of 5.6% under perioperative anticoagulation. Our study provides essential knowledge for preventing or reversing progressive visual loss in CVST via ONSF.
Visual loss in CVST is often insidious, manifesting as transient amaurosis, blurred vision, and mild visual impairment in early stages of disease. If prolonged, ischemia or optic nerve compression potentially progresses and manifests as visual-acuity loss and/or visual-field defects. Although the International Study on Cerebral Vein and Dural Sinus Thrombosis revealed that the visual impairment in CVST isn’t severe, its frequency may be underestimated owing to the sole inclusion of severe visual loss (< 20/50) [6] and the use of the confrontation testing rather than the automated perimetry or optometric evaluation [6, 7]. Recently, Liu et al. [3] found that 40% of patients experienced permanent visual-field deficits detected by automated perimetry. They also found that 21.5% of patients demonstrated worsening papilledema on follow-up examinations despite maximum treatment. These patients were at high risk of vision loss with worsening papilledema in chronically elevated ICP [3]. Similarly, another study demonstrated that, at the 1-year follow-up, 51.7% of patients presented with worse ocular presentation than onset, and 22.4% of them presented with severe vision loss due to optic atrophy [5]. In our study, all patients continued to exhibit varying degrees of BCVA and/or VFs impairment, despite adequate anticoagulation and ICP-lowering therapy: 11 of 18 patients (61.1%) presented with a preoperative BCVA worse than 20/800, and 6 of 18 patients (33.3%) presented with “NLP.” Thus, visual functions of patients should be closely monitored, and visual impairment actively prevented and treated.
The pathogenesis of visual loss in CVST involves venous infarction and compression from secondary IH due to impaired CSF absorption [1]. Elevated ICP transmitted to the optic nerve subarachnoid space leads to optic nerve sheath hypertension and impairs axoplasmic transport and blood supply to the optic nerve [3, 18, 19]. In our study, all patients experienced an ICP > 300 mmH2O, except for one patient (no. 5) with an ICP of 210 mmH2O.
Treatment options for CVST-induced visual loss in IH range from conservative to surgical. Conservative strategies include ICP-lowering medications (acetazolamide, mannitol, and furosemide) and serial lumbar punctures [2]. Acetazolamide, a carbonic anhydrase inhibitor, potentially reduces ICP by lowering CSF production; nonetheless, its effect is limited [20]. Two diuretics, mannitol and furosemide, can reduce ICP via osmotic dehydration. However, excessive dehydration potentially leads to blood concentration and carries the theoretical risk of thrombosis [1]. Serial lumbar punctures may be necessary when hypertension is persistent; nevertheless, lumbar puncture typically requires temporary cessation of anticoagulants, with an attendant risk of thrombosis [20]. Despite these measures, some patients may continue experiencing progressive visual loss, like in our study [3–6]. Surgery, including shunting procedures and ONSF, is usually reserved for patients who have failed conservative therapy. No consensus has been reached regarding the optimal surgical treatment, and the choice of surgical intervention is largely dependent on local availability and expertise [21]. A systemic review revealed that shunting is ineffective in preventing death or severe disability in acute CVST [22]. Additionally, the safety of shunting is also a concern, as it can be complicated by stroke, intracranial hemorrhage, catheter malposition, CSF infection, and secondary Chiari malformation [23].
On this premise, ONSF seems to have an advantageous edge. It involves cutting slits or windows in the optic nerve sheath to allow CSF to escape and relieve optic nerve sheath hypertension-induced optic nerve injury [18, 19]. It effectively protects visual function secondary to optic nerve sheath hypertension, such as in patients with IIH and related conditions [10, 23]. Compared to shunting, ONSF has been reported to have advantages of higher rates of vision improvement, fewer complications, faster recovery, lower costs, and shorter operative time [24, 25]. In our study, the operative time was approximately 30–40 min per eye. Furthermore, we found an efficacy of 80.6% in stabilizing and/or improving visual function 1 week postoperatively and 60.7% after long-term follow-up. Since the visual function of CVST is mainly affected by two factors: (1) venous ischemia directly caused by CVST and (2) IH secondary to impaired CSF drainage, we speculated that the difference of efficacy between short- and long-term follow-up may be resulted from continued visual loss due to persistent venous ischemia.
It should be noted that we defined both the improvement and maintenance of BCVA as “Effectiveness”, which is because all the patients included in the present study were experiencing progressive visual loss despite adequate anticoagulation and ICP-lowering therapy before ONSF. Therefore, the maintenance of BCVA after ONSF indicates that ONSF may halt this deteriorating process, and could be considered effective in hindering the progressive visual loss in CVST. Noteworthily, among eight eyes with an “NLP” status before ONSF, four improved to “LP,” “HM,” or “CF” status soon after ONSF (patient NO.7, 15, 16), although another four were still in “NLP” status, potentially attributable to irreversible optic nerve damage. This indicates that ONSF plays a positive role in relieving the pressure around the optic nerve and in resolving severe visual loss in CVST patients.
It was reported that the visual acuity required for visual field examination is over 20/200, and for patients with poor central visual acuity the visual field cannot be accurately and precisely detected [26]. Similarly, in our study, 11 patients failed to undergo preoperative and postoperative VFs examinations due to severe visual impairment and even blindness. Therefore, we only presented the VFs as supplementary results to visual acuity. An interesting phenomenon is that, compared with BCVA, the visual field did not show any significant changes in the follow-ups. Our finding is consistent with the results of study by Liu [3]. They found that only 7.7% patients had decreased visual acuity, while 40% had permanent visual field deficits, which may mean that visual field defects are more serious in CVST than visual acuity loss. There are two possible reasons for this phenomenon: (1) there is selection bias because only patients with good vision have VFs, and for patients with poor central vision, the VFs cannot be detected, and (2) the peripheral visual field is more susceptible to compressive ischemia than the central visual acuity. This leads to the peripheral visual field being first damaged from the compression by IH, while the central visual acuity might remain intact [19].
Many studies have verified ONSF safety in IIH, especially after 2000, with surgical procedures development and new instruments application. Compared with the relatively high complication rates of 44.7% and 45.3% reported, respectively, in 1993 [27] and 2000 [10], the complication rate decreased significantly, ranging from 2.6 to 23.0% [28, 29]. Most complications thereafter have been transient and benign without severe sequelae, as summarized in Table 3 [10–13, 27–29]. Moreover, ONSF appears to have a lower major complication rate of 2% compared with shunting, with a major complication rate of 8%, according to a meta-analysis of medically refractory IIH [25].
Although ONSF is effective and safe, it targets the compression of optic nerve secondary to impaired CSF drainage and IH. We emphasize that the anticoagulant is still a very basic and important treatment for venous ischemia directly caused by CVST, whereas ONSF can be considered an adjunctive treatment for progressive vision loss of CVST patients in combination with anticoagulation and ICP-lowering therapy. However, concerns regarding the use of perioperative anticoagulants have limited the application of ONSF in CVST. Whether to continue anticoagulation treatment perioperatively is a common clinical dilemma, and surgeons must balance vision-threatening hemorrhage risk against life-threatening thrombo-embolic events. This study used perioperative anticoagulation and bridging strategies to investigate ONSF safety under perioperative anticoagulation for CVST. The results showed a low complication rate of 5.6% without hemorrhagic events. This indicates that ONSF for CVST under perioperative anticoagulation is safe and practicable. Likewise, our perioperative anticoagulation and bridging strategies might be applied to lumber puncture, which usually accompanies a similar anticoagulation dilemma [30]. Notwithstanding, further studies are warranted.
Although preoperative questionnaire was not administered, most patients were in extreme frustration, anxiety, and desperation upon admission because of fulminant progressive visual loss. Visual function cannot fully reflect psychological distress, social isolation, fear, or negative effects on daily life. The ADL and VFQ-25 evaluations were added at the final follow-up to multidimensionally assess the impact of visual problems on patients. ADL results revealed that most patients maintained basic self-care ability in life, while VFQ-25 results indicated that their QoL was significantly affected by visual loss, especially regarding dependency, driving, distance activity, social function, and mental health.
Notably, these patients had visited three to four hospitals before receiving ONSF, and five (27.8%) had a vision-loss duration of over 6 months. Prolonged IH and delayed ONSF treatment appeared to negatively impact visual function [24], we recommend that clinicians might consider ONSF earlier for patients with progressive visual loss. In future investigations, a prospective, randomized controlled trial comparing surgical treatment options (including ONSF and shunting) for CVST-induced visual loss is required. The biggest strength of our study is that we are the first to report the long-term follow-up results of ONSF in a larger population of CVST-induced progressive visual loss than previously studied, while anticoagulants were used simultaneously. However, our study has a few limitations. First, it was subject to selection bias during data collection. The 18 patients we retrieved were referred to our ophthalmology department from neurology departments of various hospitals, and they all had severe optic nerve damages on admission. Second, although it did not affect our diagnosis of IH, the ICP in a portion of patients was recorded as over 330 mmH2O, for it exceeded the maximal column manometer used in the neurology departments. Third, for the convenience of patients, the long-term follow-ups were mainly conducted by doctors from local hospitals. These results of ophthalmological examinations were mainly collected and transmitted via the Internet, and the questionnaires were performed by Internet or telephone, which might cause bias. Fourth, in the present study, we mainly evaluated visual functional parameters such as BCVA and VFs, as well as fundoscopy findings, other structural or functional assessments such as visual-evoked potentials, quantitative OCT, and recanalization of sinus in MRI/MRV were not conducted, which will be worth investigation in future research. Fifth, due to poor visual acuity or lack of compliance, 11 patients failed to undergo preoperative and postoperative VFs examinations. Finally, this was a retrospective, nonrandomized, noncomparative study with a relatively small sample size. In the future, a large-scale prospective, randomized, controlled trial comparing different treatment options for CVST-induced visual loss is required.
Conclusions
Conclusively, progressive visual loss remains a big challenge in CVST, which deserves more attention from neurologists, ophthalmologists, and neurosurgeons. Considering its favorable efficacy and safety, ONSF can be considered an important approach to resolving progressive visual loss in CVST combined with anticoagulation and ICP-lowering therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
This research was supported by the National Basic Research Program of China (973 Program, grant no.: 2015CB554103), Department of Science and Technology of Sichuan Province, China (grant no.: 2020YFSY0044), the Natural Science Foundation of Shanghai (grant no.: 22ZR1449800), National Natural Science Foundation of China (grant no.: 81271035, 81401038 and 82000880). The journal’s Rapid Service Fee was provided by the authors.
Authorship
All named authors meet the ICMJE criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
HC and YXG conceived and designed the study. HC, YX, QS, JZ, LB, TF, ST, HW, BLD and QL acquired the data. ZSL, LC, YX and QS analyzed data and designed the figures. HC, YXG, ZSL, LC, YX and QS accessed and verified the underlying data. ZSL and LC drafted the original manuscript. HC and YXG critically reviewed and revised the manuscript. All authors gave final approval of the version to be published. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclosures
Zongshan Li, Lu Cheng, Yang Xu, Qiao Sun, Jian Zhang, Lin Bai, Ting Feng, Song Tan, Huan Wang, Bolin Deng, Qiang Li, Yaxing Gui and Hui Chen declare that they have no conflict of interest.
Compliance with Ethics Guidelines
The current study was approved by the Medical Ethical Committee of the Shanghai General Hospital (2020-N-145). Each patient provided written informed consent prior to be included in the study. The study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Zongshan Li, Lu Cheng, Yang Xu and Qiao Sun are co-first authors of this manuscript.
Contributor Information
Yaxing Gui, Email: YaxingGui@shsmu.edu.cn.
Hui Chen, Email: 352477354@qq.com.
References
- 1.Ropper AH, Klein JP. Cerebral venous thrombosis. N Engl J Med. 2021;385:59–64. doi: 10.1056/NEJMra2106545. [DOI] [PubMed] [Google Scholar]
- 2.Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6:162–170. doi: 10.1016/S1474-4422(07)70029-7. [DOI] [PubMed] [Google Scholar]
- 3.Liu KC, Bhatti MT, Chen JJ, et al. Presentation and progression of papilledema in cerebral venous sinus thrombosis. Am J Ophthalmol. 2020;213:1–8. doi: 10.1016/j.ajo.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Ferro JM, Lopes MG, Rosas MJ, Ferro MA, Fontes J, Cerebral Venous Thrombosis Portugese Collaborative Study Group Cerebral Venous Thrombosis Portugese Collaborative Study Group. Long-term prognosis of cerebral vein and dural sinus thrombosis. results of the VENOPORT study. Cerebrovasc Dis. 2002;13:272–278. doi: 10.1159/000057855. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Fang B, Wei S. Analysis of clinical features of ocular presentation in cranial venous sinus thrombosis. Eur J Med Res. 2011;16:324–327. doi: 10.1186/2047-783X-16-7-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F, ISCVT Investigators Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) Stroke. 2004;35:664–670. doi: 10.1161/01.STR.0000117571.76197.26. [DOI] [PubMed] [Google Scholar]
- 7.Ferro JM, Canhão P, Stam J, et al. Delay in the diagnosis of cerebral vein and dural sinus thrombosis: influence on outcome. Stroke. 2009;40:3133–3138. doi: 10.1161/STROKEAHA.109.553891. [DOI] [PubMed] [Google Scholar]
- 8.Ding JY, Zhou D, Geng TT, et al. To predict visual deterioration according to the degree of intracranial hypertension in patients with cerebral venous sinus thrombosis. Eur Neurol. 2018;80:28–33. doi: 10.1159/000492184. [DOI] [PubMed] [Google Scholar]
- 9.Kristoffersen ES, Harper CE, Vetvik KG, Zarnovicky S, Hansen JM, Faiz KW. Incidence and mortality of cerebral venous thrombosis in a norwegian population. Stroke. 2020;51:3023–3029. doi: 10.1161/STROKEAHA.120.030800. [DOI] [PubMed] [Google Scholar]
- 10.Banta JT, Farris BK. Pseudotumor cerebri and optic nerve sheath decompression. Ophthalmology. 2000;107:1907–1912. doi: 10.1016/S0161-6420(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 11.Moreau A, Lao KC, Farris BK. Optic nerve sheath decompression: a surgical technique with minimal operative complications. J Neuroophthalmol. 2014;34:34–38. doi: 10.1097/WNO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 12.Chandrasekaran S, McCluskey P, Minassian D, Assaad N. Visual outcomes for optic nerve sheath fenestration in pseudotumour cerebri and related conditions. Clin Exp Ophthalmol. 2006;34(7):661–665. doi: 10.1111/j.1442-9071.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 13.Obi EE, Lakhani BK, Burns J, Sampath R. Optic nerve sheath fenestration for idiopathic intracranial hypertension: a seven year review of visual outcomes in a tertiary centre. Clin Neurol Neurosurg. 2015;137:94–101. doi: 10.1016/j.clineuro.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Murdock J, Tzu JH, Schatz NJ, Lee WW. Optic nerve sheath fenestration for the treatment of papilledema secondary to cerebral venous thrombosis. J Neuroophthalmol. 2014;34:67–69. doi: 10.1097/WNO.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 15.Elnahry AG, Talbet JH, El Mahgoub IR, Elnahry GA. Optic nerve sheath fenestration for papilledema due to cerebral venous sinus thrombosis associated with antiphospholipid syndrome: a case report. Am J Case Rep. 2021;22:e930497. doi: 10.12659/AJCR.930497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suárez-Dono J, Cervantes-Pérez E, Pena-Seijo M, et al. CRONIGAL: prognostic index for chronic patients after hospital admission. Eur J Intern Med. 2016;36:25–31. doi: 10.1016/j.ejim.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item national eye institute visual function questionnaire. Arch Ophthalmol. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 18.Tso MO, Hayreh SS. Optic disc edema in raised intracranial pressure. IV. Axoplasmic transport in experimental papilledema. Arch Ophthalmol. 1977;95:1458–62. [DOI] [PubMed]
- 19.Hayreh SS. Pathogenesis of optic disc edema in raised intracranial pressure. Prog Retin Eye Res. 2016;50:108–144. doi: 10.1016/j.preteyeres.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saposnik G, Barinagarrementeria F, Brown RD, Jr, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–1192. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 21.Piper RJ, Kalyvas AV, Young AM, Hughes MA, Jamjoom AA, Fouyas IP. Interventions for idiopathic intracranial hypertension. Cochrane Database Syst Rev. 2015;2015:CD003434. doi: 10.1002/14651858.CD003434.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobo S, Ferro JM, Barinagarrementeria F, ISCVT Investigators et al. Shunting in acute cerebral venous thrombosis: a systematic review. Cerebrovasc Dis. 2014;37:38–42. doi: 10.1159/000356524. [DOI] [PubMed] [Google Scholar]
- 23.Jefferis JM, Littlewood RA, Pepper IM, Hickman SJ, Salvi SM. Optic nerve sheath fenestration via a supero-medial eyelid skin crease approach for the treatment of idiopathic intracranial hypertension in a UK population. Eye (Lond) 2021;35:1418–1426. doi: 10.1038/s41433-020-1024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalyvas AV, Hughes M, Koutsarnakis C, et al. Efficacy, complications and cost of surgical interventions for idiopathic intracranial hypertension: a systematic review of the literature. Acta Neurochir (Wien) 2017;159:33–49. doi: 10.1007/s00701-016-3010-2. [DOI] [PubMed] [Google Scholar]
- 25.Satti SR, Leishangthem L, Chaudry MI. Meta-analysis of CSF diversion procedures and dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2015;36:1899–1904. doi: 10.3174/ajnr.A4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 27.Plotnik JL, Kosmorsky GS. Operative complications of optic nerve sheath decompression. Ophthalmology. 1993;100:683–690. doi: 10.1016/S0161-6420(93)31588-5. [DOI] [PubMed] [Google Scholar]
- 28.Vaidya NS, Mahmoud AM, Buzzacco D, Katz SE. Visual outcomes following optic nerve sheath fenestration via the medial transconjunctival approach. Orbit. 2016;35:271–277. doi: 10.1080/01676830.2016.1193530. [DOI] [PubMed] [Google Scholar]
- 29.Bersani TA, Meeker AR, Sismanis DN, Carruth BP. Pediatric and adult vision restoration after optic nerve sheath decompression for idiopathic intracranial hypertension. Orbit. 2016;35:132–139. doi: 10.1080/01676830.2016.1176051. [DOI] [PubMed] [Google Scholar]
- 30.Ruff RL, Dougherty JH., Jr Complications of lumbar puncture followed by anticoagulation. Stroke. 1981;12:879–881. doi: 10.1161/01.STR.12.6.879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.