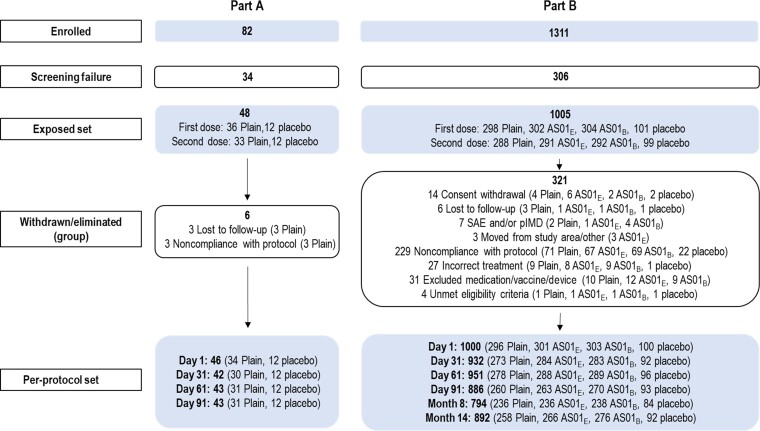
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of participant cohorts in study parts A and B. Abbreviations: AS01B and AS01E, adjuvanted vaccine formulations with the corresponding vaccine adjuvant systems; pIMD, potential immune-mediated disease; Plain, unadjuvanted vaccine formulations; RSVPreF3, prefusion conformation of the respiratory syncytial virus F protein; SAE, serious adverse event.