Abstract
Antimicrobial Resistance (AMR) is a growing global health challenge that threatens to undo gains in human and animal health. Prevention and control of AMR requires functional antimicrobial stewardship (AMS) program, which is complex and often difficult to implement in low- and middle-income countries. We aimed to describe the processes of establishing and implementing an AMS program at Connaught Hospital in Sierra Leone. The project involved the setting up of an AMS program, capacity building and performing a global point prevalence survey (GPPS) at Sierra Leone’s national referral hospital. Connaught Hospital established a multidisciplinary AMS subcommittee in 2021 to provide AMS services such as awareness campaigns, education and training and review of guidelines. We performed a GPPS on 175 patients, of whom more than half (98, 56.0%) were prescribed an antibiotic: 63 (69.2%) in the surgical wards and 53 (51.2%) in the medical wards. Ceftriaxone (60, 34.3%) and metronidazole (53, 30.3%) were the most common antibiotics prescribed to patients. In conclusion, it is feasible to establish and implement an AMS program in low-income countries, where most hospitalized patients were prescribed an antibiotic.
Keywords: global point prevalence survey, GPPS, antimicrobial stewardship program, AMS program, AMS team, Freetown, Sierra Leone
1. Introduction
Antimicrobial Resistance (AMR) is a growing global health challenge that threatens to undo gains in human and animal health [1,2]. A recent global estimate reports that 4.95 million deaths annually are associated with AMR, of which 1.27 million are directly attributable to bacterial AMR [3]. By 2050, an estimated 10 million AMR-related deaths will occur each year, many in Africa and Asia, if action is not taken to address the problem [4].
To stem the growing burden of AMR, the World Health Organization (WHO) developed a global action plan in 2015 to support the AMR prevention and control efforts of its member states [2]. Among the five strategic principles of the global action plan, WHO recommends rational use of antimicrobials to prevent the development of resistance [2]. Antimicrobial Stewardship (AMS) is a major tool that combines interventions designed to ensure the appropriate use of antimicrobials to contain the emergence of resistance and maintain the efficacy of existing antimicrobials [4]. Establishing a functional AMS program is essential to reduce unnecessary antimicrobial use and thus the burden of AMR [5,6,7]. Despite the need for AMS, its implementation in low- and middle-income countries (LMICs) is complex and often difficult to achieve because AMS implementation is influenced by the local context of the weak health systems in these countries [5,6,7].
Sierra Leone, a low-income country in West Africa, is struggling to rebuild its health system following the adversity of the 2014–2016 Ebola outbreak and a decade-long civil war from 1991 to 2002 [8,9,10]. In 2018, Sierra Leone developed a National Strategic Plan to combat AMR, which is aligned with the WHO Global Action Plan [2,11]. Yet, there are no functional stewardship activities in any of the hospitals in Sierra Leone.
This situation, and the fact that our previous work reported many challenges with rational antibiotic prescribing prompted us to set up the only existing AMS program at Sierra Leone’s national referral hospital [12,13]. In this article, we aim to describe the processes of establishing and operationalizing an AMS program in the national referral hospital of Sierra Leone.
2. Results
2.1. Setting up and Operationalizing an AMS Program in a Low-Income Country
Connaught Hospital established an AMS subcommittee in 2021 to provide input to the Infection Prevention and Control (IPC), Water Sanitation and Hygiene (WASH) and AMS committee (this broader committee existed before but did not include AMS). Members of the subcommittee come from across the hospital, including nursing, medicine, pharmacy, laboratories, surgery and IPC. The subcommittee nominated an AMS lead and deputy. The terms of reference were agreed and the subcommittee aimed to meet every month. The hospital’s IPC and WASH committee incorporated AMS into its remit, and agreed that the lead for the AMS subcommittee would reports to the broader IPC, WASH and AMS committee on a quarterly basis, which subsequently feeds back to the senior management of the hospital.
The AMS Subcommittee supported the conduct of the GPPS and used the results as the basis for an AMS action plan. This subcommittee provides direction and oversight for the promotion and delivery of successful AMS services throughout the institution.
The subcommittee supports Connaught Hospital in identifying and implementing any national guidance on AMS, including identifying any resources needed to overcome constraints. The subcommittee also led hospital awareness campaigns and partnered with the nursing education team to launch the use of an AMS board game to raise awareness and educate nursing staff. In addition, the AMS subcommittee led the planning of educational events related to AMS, drawing on experts to speak, designing messaging and inviting stakeholders.
2.2. Designing AMS Related Policies and Guidelines
The IPC, WASH, and AMS broader committee developed terms of reference for the AMS subcommittee to support and define its operations. The need to review the surgical prophylaxis guidelines was identified through previous research at the hospital and the recent GPPS results [12,13]. It was decided to bring in various stakeholders for the review of the surgical prophylaxis guidelines. Stakeholders included representatives from Connaught Hospital, King’s Global Health Partnerships (KGHP), the WHO Country Office and Ministry of Health and Sanitation. The review of surgical prophylaxis guidelines for Connaught Hospital was conducted with the view to creating a national guideline which can be shared and implemented across all government hospitals/facilities offering surgical services in Sierra Leone. The involvement of key stakeholders enabled the validation and promotion of these guidelines at national level. Given that most of the relevant expertise for AMR is based in the capital of Sierra Leone, creation of a guideline that is applicable to other facilities, is evidence of a commitment to spread the benefits from this AMS program across the country.
We developed a five-year AMS action plan that is based on five strategic goals, including leadership commitment, AMS actions, education and training, monitoring and surveillance, and reporting feedback in healthcare settings.
2.3. Partnership and Funding
Connaught Hospital has an established long-term partnership with an international non-governmental organization, KGHP. Connaught Hospital, in collaboration with KGHP, the Young Pharmacists Group and the Pharmaceutical Society of Sierra Leone, secured funding from the Commonwealth Partnerships for Antimicrobial Stewardship. These partnerships are funded by the Fleming Fund (a UK aid program) and managed by the Tropical Health and Education Trust (THET) and the Commonwealth Pharmacists Association (CPA). This grant supported the work described. The hospital also leverages the activities of existing partnerships within the Ministry of Health and Sanitation and international health agencies such as the WHO.
2.4. AMS Champion Training
During the program, ten pharmacists (5 women; 5 men) were recruited and trained as AMS Champions, using Connaught Hospital as a training site.
Those recruited to the training program work in the pharmacy sector, including hospitals, community pharmacies, and administrative departments. The training program design was based on the WHO competency framework and curricula for healthcare workers and accredited by the Pharmaceutical Society of Sierra Leone [14]. This blended learning program included a range of learning activities: ward visits; group discussions; individual discussions with tutors about patient cases/AMS; case presentations and discussions; preparing, collecting data for, and analyzing the GPPS; community awareness raising activities; online continuous professional development (CPD) modules; and the AMS board game.
The AMS Champions together with the AMS Lead and Deputy led the data collection for the GPPS and supported the subcommittee in raising awareness about AMS and disseminating the GPPS findings in the hospital.
2.5. Global Point Prevalence Survey (GPPS)
During the course of the project, a GPPS was performed on 175 inpatients in the hospital. Their mean (SD) age was 33 ± 19 years. The majority were males (124, 70.9%), admitted to the surgical wards of the hospital (112, 64.0%) and aged 20–40 years (79, 45.1%).
More than half (98, 56.0%) of the patients were prescribed an antibiotic, most in the surgical wards (63, 69.2%). Antibiotics prescribed to patients in this study were mainly in the Access (85, 48.6%) or Watch (83, 47.4%) groups. The most common reason for prescribing antibiotics was to treat community-acquired infections (92, 52.5%) (Table 1). Ceftriaxone J01DD04 (60, 34.3%) and metronidazole P01AB01 (53, 30.3%) were the most common antibiotics prescribed to patients in this study (Table 2). Twenty-five (14.2%) cases did not specify the indications for antibiotic prescription. Only 25 (15.3%) patients were prescribed antibiotics according to hospital treatment guidelines.
Table 1.
Demographic characteristics of participants and antibiotics prescribing patterns.
| Parameter | Total | MW | SW | ICU |
|---|---|---|---|---|
| n(%) | n (%) | n (%) | n(%) | |
| Overall total | 175 (100) | 60 (34.3) | 112 (64.0) | 3 (1.7) |
| Sex | ||||
| Female | 51 (29.1) | 30 (50.0) | 18 (16.1) | 3 (100.0) |
| Male | 124 (70.9) | 30 (50.0) | 94 (83.9) | 0 (0.0) |
| Age | ||||
| <20 | 45 (25.7) | 7 (11.7) | 38 (33.9) | 0 (0.0) |
| 20–40 | 79 (45.2) | 29 (48.3) | 49 (43.8) | 1 (33.3) |
| 41–60 | 27 (15.4) | 14 (23.3) | 13 (11.6) | 0 (0.0) |
| >60 | 24 (13.7) | 10 (16.7) | 12 (10.7) | 2 (66.7) |
| Mean (SD) | 33 (18.0) | - | - | - |
| Prescriptions based on laboratory results | ||||
| Yes | 19 (10.9) | 5 (8.3) | 14 (12.5) | 0 (0.0) |
| No | 156 (89.1) | 55 (91.7) | 98 (87.5) | 3 (100) |
| Indications for prescribing antibiotics | ||||
| CAI | 92 (52.5) | 37 (61.7) | 52 (46.4) | 0 (0.0) |
| HAI | 30 (17.1) | 16 (26.7) | 22 (19.6) | 3 (100.0) |
| Medical prophylaxis | 1 (0.6) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Surgical prophylaxis | 28 (16.0) | 0 (0.0) | 28 (25.0) | 0 (0.0) |
| Unknown | 24 (13.7) | 8 (13.3) | 16 (14.3) | 0 (0.0) |
| Documented antibiotic stop/review date | ||||
| Yes | 38 (21.7) | 16 (26.7) | 22 (19.6) | 0 (0.0) |
| No | 137 (78.3) | 44 (73.3) | 90 (80.4) | 3 (100) |
| Reason in notes | ||||
| Yes | 119 (68.0) | 40 (66.7) | 76 (67.9) | 3 (100) |
| No | 56 (32.0) | 20 (33.3) | 36 (32.1) | 0 (0.0) |
| AWaRe | ||||
| Access | 85 (48.6) | 26 (43.3) | 58 (51.8) | 1 (33.3) |
| Watch | 83 (47.4) | 29 (48.3) | 52 (46.4) | 2 (66.7) |
| Not classified | 7 (4.0) | 5 (8.3) | 2 (1.8) | 0 (0.0) |
| Prevalence of antibiotic use | 98 (56.0) | 33 (51.6) | 63 (69.2) | 2 (100.0) |
| Dose per day (IQR) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2.5 (2–3) |
| Route | ||||
| Parenteral | 130 (73.9) | 38 (63.3) | 88 (78.6) | 4 (100) |
| Oral | 46 (26.1) | 22 (36.7) | 24 (21.4) | 0 (0) |
MW = Medical ward; SW =Surgical ward; ICU = Intensive Care Unit. CAI = Community acquired infections; HAI =Healthcare Infections; IQR = Interquartile range.
Table 2.
Frequency of administration of antibiotics and AWaRe category.
| Variable | ATC Code | AWaRe Category |
Drug Administration | |||
|---|---|---|---|---|---|---|
| Total n (%) |
MW n (%) |
SW n (%) |
ICU N (%) |
|||
| Acyclovir | - | - | 1 (0.6) | 1 (1.7) | - | - |
| Amoxicillin | J01CA04 | Access | 18 (10.3) | 12 (20.0) | 6 (5.4) | - |
| Amoxicillin-clavulanate | J01CR02 | Access | 8 (4.6) | 3 (5.0) | 5 (4.5) | - |
| Ampicillin | J01CA01 | Access | 1 (0.6) | - | 1 (0.9) | - |
| Ampicillin-cloxacillin | J01CR50 | Access | 2 (1.1) | - | 2 (1.8) | - |
| Artemether-lumefantrine | - | - | 2 (1.1) | 2 (3.3) | - | - |
| Artesunate | - | - | 1 (0.6) | 1 (1.7) | - | - |
| Azithromycin | J01DB01 | Watch | 8 (4.6) | 8 (13.8) | - | - |
| Cefepime | J01DE01 | Watch | 1 (0.6) | - | 1 (0.9) | - |
| Cefoxitin | J01DC01 | Watch | 1 (0.6) | 1 (1.7) | - | - |
| Cefixime | J01DD08 | Watch | 1 (0.6) | - | 1 (0.9) | - |
| Ceftriaxone | J01DD04 | Watch | 60 (34.3) | 15 (25.0) | 43 (38.4) | 2 (66.7) |
| Clarithromycin | J01FA09 | Watch | 12 (1.2) | 1 (1.7) | 1 (0.9) | - |
| Erythromycin | J01FA01 | Watch | 2 (1.1) | - | 2 (1.8) | - |
| Flucloxacillin | J01CF05 | Access | 2 (1.1) | 2 (3.3) | - | - |
| Fluconazole | - | - | 2 (1.1) | 1 (1.7) | - | - |
| Gentamycin | J01GB03 | Access | 2 (1.1) | - | 2 (1.8) | - |
| Levofloxacin | J01MA12 | Watch | 4 (2.3) | 2 (3.3) | 2 (1.8) | - |
| Metronidazole | P01AB01 | Access | 53 (30.3) | 9(15.5) | 43 (38.4) | 1 (33.3) |
| Ofloxacin | J01MA01 | Watch | 1 (0.6) | - | 1 (1.7) | - |
| Piperacillin-tazobactam | J01CR05 | Watch | 1 (0.6) | - | 1 (0.9) | |
| TDF+3TC+DTG | - | Watch | 1 (0.6) | 1 (1.7) | - | - |
| Tinidazole | P01AB02 | Access | 22 (2.2) | 22 (7.3) | 1 (0.9) | - |
| Tinifloxacin | J01RA13 | Access | 2 (0.2) | 2 (0.7) | - | - |
| Vancomycin | J01XA01 | Watch | 1 (0.6) | - | 1 (0.9) | |
MW = Medical ward; SW =Surgical ward; ICU = Intensive Care Unit; TDF + 3TC + DTG = Tenofovir + Lamivudine + Dolutegravir; ATC = Anatomic Therapeutic and Chemical classification of drugs.
In multivariable regression analysis, women were less likely to have an indication for prescribing antibiotics: [aOR 0.28, 95% CI (0.09–0.83); p = 0.022]. Indications for prescribing were less likely to be stated for the unclassified antimicrobial agents than Access antibiotics (Table 3).
Table 3.
Multivariable regression of presence of prescription indication.
| Parameter | Indication for Antibiotic Prescription | aOR (95% CI) | p | |
|---|---|---|---|---|
| Yes (%) | No (%) | |||
| Age * | 32 (20) | 38 (16) | 0.98 (0.96–1.01) | 0.221 |
| Sex | ||||
| Male | 111 (73.5) | 13 (52) | 1 | - |
| Female | 40 (26.5) | 12 (48) | 0.28 (0.09–0.83) | 0.022 |
| Ward | ||||
| Medical | 52 (34.4) | 8 (32) | 1 | - |
| Surgical | 96 (63.6) | 16 (64) | 0.47 (0.15–1.45) | 0.188 |
| ICU | 3 (2.0) | 1 (4) | 0.76 (0.06–9.47) | 0.829 |
| Reason in notes | ||||
| No | 43 (28.5) | 14 (56) | 1 | - |
| Yes | 108 (71.5) | 11 (44) | 3.78 (1.47–9.77) | 0.006 |
| Prescription based on lab. results | ||||
| No | 137 (90.7) | 20 (80) | ||
| Yes | 14 (9.3) | 5 (20) | 0.25 (0.07–0.91) | 0.035 |
| AWaRe | ||||
| Access | 73 (44.3) | 12 (48) | 1 | |
| Watch | 74 (49.0) | 9 (36) | 1.25 (0.46–3.38) | 0.658 |
| Not classified | 4 (2.7) | 4 (16) | 0.16 (0.03–0.84) | 0.031 |
* Reported as mean (standard deviation); ICU = intensive care unit.
3. Discussion
We report our experience in establishing and operationalizing an AMS program in Sierra Leone to add to the evidence base in the global literature. The implementation and operationalization of an AMS program in hospitals of low-income countries is important because AMS activities are critical to the achievement of the United Nations Sustainable Development Goals (Targets 3.1 to 3.3 and 3.8, and Goal 6), especially those focused on reducing AMR [15]. This report of the activities, for the only existing hospital AMS program in Sierra Leone at the moment, showed that it is feasible to establish and implement an AMS program in low-income countries when there is support from the local human resources. An AMS program was previously set up in a rural hospital in Sierra Leone but it ceased to exist after the departure of the foreign experts who carried out its activities, demonstrating that a sustainable AMS program is the one that receives support from the local hospital structures [16].
Research from hospitals across Sierra Leone, including Connaught Hospital, found deep-rooted challenges not only in antimicrobial prescribing and antibiotic resistance, but also in IPC [17,18,19,20,21,22]. This reinforces the fact that an AMS program should constitute a broad-based membership that includes IPC practitioners [23]. Furthermore, we reflect on the call in 2022 by the WHO Director-General to prioritize IPC as a key to health system strengthening and universal health coverage instrument and the idea that ‘every infection prevented is an antibiotic resistance avoided’ [24]. Consequently, we adopted a multidisciplinary approach in the selection of the AMS subcommittee members and in the implementation of the AMS activities, similar to the recommendations made by Kirchhelle et al. [25]. Our AMS program is unique in that it operates within the remit of a broader IPC, WASH and AMS committee that interweaves IPC, WASH and AMS activities within an integrated framework at the hospital. Through this broader committee, the three subcommittees can share experiences, gain a better understanding of the operations of the different subcommittees, and generally improve the outcomes of their interventions.
We developed policies, updated guidelines and trained healthcare workers to support the implementation of our AMS program. In his guide on how to start a hospital AMS program, Mendelson recommends training and resource mobilization as part of the preparation for implementing AMS activities [23].
Our AMS program conducted a GPPS, which was critical in the external validation of our AMS experience, because it was easy to complete and the data collection software automatically generated a report which enabled comparison of antimicrobial prescribing at Connaught Hospital with other hospitals in the African continent and worldwide. In this hospital-wide GPPS, 56% of hospitalized patients were prescribed antibiotics, compared with 82% reported in 2017 [12]. The difference in the prevalence between the two studies could be attributed to the differences in the methods applied (point prevalence vs. period prevalence) and ongoing AMS activities or perhaps it could be due to the availability of more medical specialists to oversee antibiotic prescribing. Likewise, the departure of low-level medical staff known as community health officers (CHOs) who historically prescribed large quantities of antibiotics in the emergency department of Connaught Hospital could explain the difference in prevalence between the two studies. Yet, this prevalence of 56% is still higher than the African average of 42% [26]. In addition, antibiotic use remained higher among inpatients in adult surgical wards (69.6%) compared with adult medical wards (51.9%). This discrepancy may be related to the surgical team’s concern about poor IPC and environmental conditions in the wards or operating theatres leading to unnecessary and prolonged use of prophylactic antibiotics. We join the call to reshape the global health architecture to reduce reliance on the use of antibiotics for patient care and encourage non-medicinal care, such as providing supports to patients and addressing their concerns [27]. Combined with the results of a previous study, this point prevalence survey showed an ongoing high rate of antibiotic use among surgical patients and provided the stimulus for reviewing and updating guidelines for antibiotic prophylaxis in surgery [13]. We believe that the inappropriate use of antibiotics in the surgical wards will reduce with the implementation of targeted AMS activities.
Although no Reserve antibiotics were prescribed in this study, which may reflect unavailability, the prescription of non-antibacterial agents such artesunate and acyclovir without documented indication calls for expanded stewardship activities.
Our AMS program is not without challenges or limitations. One of the main challenges in the implementation of the AMS program is the unavailability of financial support from local sources as the Government of Sierra Leone does not provide specific budgetary support for AMS activities. As shown by GPPS, less than 11% of antibiotic prescriptions were supported by microbiology or other laboratory tests, showing the hospital’s limited laboratory capacity to support AMS activities. Owing to the funder’s requirements, focused primarily on pharmacists, we could not directly fulfil the multidisciplinary principles of AMS in our AMS champion training program. Nonetheless, the AMS champions provided support for certain elements such as the GPPS and spent time on the wards talking to doctors and nurses about the prescribing issues they identified. Finally, the limited availability of the AMS subcommittee staff has at times affected the conduct of the AMS program at Connaught Hospital.
4. Methods and Materials
4.1. Project Design and Settings
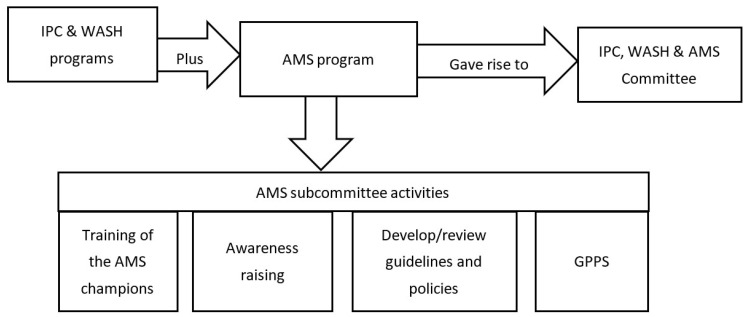
The project involved the setting up of an AMS program, capacity building and performing a point prevalence survey at the Sierra Leone’s national referral hospital for medical and surgical cases (Figure 1). Sierra Leone is divided into five geographical regions, of which the Western Area is the most densely populated with a population of approximately 1.5 million [28,29]. The public health system is divided into primary, secondary and tertiary levels of care.
Figure 1.
Flow diagram showing AMS subcommittee activities and related structures. AMS = Antimicrobial stewardship; GPPS = Global point prevalence survey; IPC = Infection Prevention and Control; WASH = Water Hygiene and Sanitation.
The project was implemented in Connaught Hospital, which is Sierra Leone’s national referral hospital in the Western Area of Sierra Leone with a capacity of 300 beds. Connaught Hospital is affiliated with the College of Medicine and Allied Health Sciences of the University of Sierra Leone. It has 12 wards, an intensive care unit, a medical observation department, and an accident and emergency department [30].
4.2. Global Point Prevalence Survey (GPPS) Data Collection and Analysis
We conducted a point prevalence survey using an online data collection tool provided by the GPPS central platform [https://www.global-pps.com/ (accessed on 20 January 2023)] to determine antibiotic prescribing patterns in the wards of Connaught Hospital on 11 March 2022. Indications for antibiotic prescription were divided into community-acquired infections, healthcare-associated infections, and medical or surgical prophylaxis. Cases for which no antibiotic prescription indication was specified were designated as unknown cases.
Upon completion of the data collection, the data was exported into a Microsoft Excel file (Microsoft, Redmond, WA, USA), cleaned, coded and then transferred into Stata Version 16 (StataCorp LLC, College Station, TX, USA) for analysis. Descriptive statistics such as mean and standard deviation were used for normal continuous variables; median and interquartile ranges were used for nonparametric variables; and frequencies and percentages were used for categorical variables. The prescribed antibiotics were categorized using the WHO’s Access, Watch and Reserve (AWaRe) framework and the Anatomic Therapeutic and Chemical Classification (ATC) System as described in the WHO’s Essential Model List [31,32].
A multivariable logistic regression model was built to predict factors associated with stated indications for antibiotic prescription. All variables were sequentially entered into the model, and if the overall model fit was improved according to the Akaike Information Criterion (AIC), all variables were retained in the final model. All tests were two-tailed and significance was set at p < 0.05.
5. Conclusions and Recommendations
In conclusion, it is feasible to establish and implement an AMS program in a low-income country such as Sierra Leone, where hospitalized patients are likely to be given antibiotics.
We recommend the following in establishing an AMS program in a low-income country:
-
(a)
Use existing structures, such as IPC committee and teams (where available).
-
(b)
Establish partnership with local and international agencies.
-
(c)
Secure funding.
-
(d)
Establish the policy environment such as terms of reference for the AMS team.
-
(e)
Establish a multidisciplinary AMS team.
-
(f)
Train healthcare workers.
-
(g)
Create an action plan to guide activities.
-
(h)
Use pre-existing GPPS methodologies to track progress of the AMS work.
Acknowledgments
The GPPS was supported by the AMS Pharmacist Champions; Sia Nusu Kabba, John Sahr Sellu, Angella Kaikai, Khadija Sesay, Paulina Jalloh, Sally Mattu Conteh, Mariama Jalloh, Abubakarr Sesay, Sheku Idriss and Christian Brainard. The project also received support from the THET Consultant, Momoh Jimmy, and the National AMR Program Focal, Joseph Sam Kanu.
Author Contributions
Conceptualization, S.L., S.T., H.L., A.J., C.B. and M.B.; methodology, S.T., S.L., H.L., N.S., I.J., C.T. and S.B. Formal analysis, E.F., S.L. and D.S.; data curation, N.S., N.K.N., I.J., C.T., S.T., S.B. and D.S.; writing—original draft preparation, S.L., S.T., M.N.P.B., H.L., G.F.D., M.S.K. and J.B.W.R. writing—review and editing, S.L., S.T., M.B., E.F., A.J. and S.B.; funding acquisition, C.B., M.S.K., M.B., G.F.D. and J.B.W.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethics approval was obtained from the Sierra Leone Ethics and Scientific Review Committee of the Ministry of Health and Sanitation, Government of Sierra Leone.
Informed Consent Statement
This is an observational study in the context of implementing a WHO-recommended program to reduce inappropriate antimicrobial prescribing at institutional level. The study procedures do not involve risk to the participating institution and therefore informed written consent was not necessary for this study. Permission to collect the data was obtained from the administrations of Connaught Hospital.
Data Availability Statement
Data is available at the University of Sierra Leone repository and will be available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project is funded by the UK Department of Health and Social Care's Fleming Fund using UK aid. The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health and Social Care.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. London: Review on Antimicrobial Resistance. 2016. [(accessed on 4 March 2022)]. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- 2.World Health Organization . Global Action Plan on Antimicrobial Resistance. WHO; Geneva Switzerland: 2015. [(accessed on 4 March 2022)]. pp. 1–28. Available online: https://www.who.int/publications/i/item/9789241509763. [Google Scholar]
- 3.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collignon P., Beggs J.J., Walsh T.R., Gandra S., Laxminarayan R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet Health. 2018;2:e398–e405. doi: 10.1016/S2542-5196(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 5.Pierce J., Apisarnthanarak A., Schellack N., Cornistein W., Maani A.A., Adnan S., Stevens M.P. Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries. Int. J. Infect. Dis. 2020;96:621–629. doi: 10.1016/j.ijid.2020.05.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox J.A., Vlieghe E., Mendelson M., Wertheim H., Ndegwa L., Villegas M.V., Gould I., Levy Hara G. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017;23:812–818. doi: 10.1016/j.cmi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 7.WHO 2019-Antimicrobial Stewardship Programmes in Healthcare Facilities in Low-and-Middle Income Countries. [(accessed on 22 July 2022)]; Available online: https://apps.who.int/iris/bitstream/handle/10665/329404/9789241515481-eng.pdf.
- 8.GoSL SLMTDP Development-Documents-National-Development-Plan-2019-23-47099. 2018. [(accessed on 14 January 2022)]. Available online: https://www.imf.org/en/Publications/CR/Issues/2019/07/09/Sierra-Leone-Economic-Development-Documents-National-Development-Plan-2019-23-47099.
- 9.Kaldor, Mary and James Vincent . Evaluation of UNDP Assistance to Conflict-Affected Countries. Case Study: Sierra Leone. United Nations Development Programme Evaluation Office; Bruxelles, France: 2006. [(accessed on 4 July 2022)]. pp. 1–39. Available online: https://cdn.sida.se/publications/files/sida48039en-sidas-support-to-undp-in-sierra-leone.pdf. [Google Scholar]
- 10.Ministry of Health and Sanitation Government of Sierra Leone Ebola Viral Disease Situation Report. [(accessed on 2 April 2022)]; Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/Ebola-Situation-Report_Vol-260.pdf.
- 11.GoSL 2018: Sierra Leone AMR Strategic Plan 2018–2022. [(accessed on 5 July 2022)]. Available online: https://www.who.int/publications/m/item/sierra-leone-national-strategic-plan-for-combating-antimicrobial-resistance.
- 12.Lakoh S., Adekanmbi O., Jiba D.F., Deen G.F., Gashau W., Sevalie S., Klein E.Y. Antibiotic use among hospitalized adult patients in a setting with limited laboratory infrastructure in Freetown Sierra Leone, 2017–2018. Int. J. Infect. Dis. 2020;90:71–76. doi: 10.1016/j.ijid.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Lakoh S., Kanu J.S., Conteh S.K., Russell J.B.W., Sevalie S., Williams C.E.E., Barrie U., Kabia A.K., Conteh F., Jalloh M.B., et al. High levels of surgical antibiotic prophylaxis: Implications for hospital-based antibiotic stewardship in Sierra Leone. Antimicrob. Steward. Healthc. Epidemiol. 2022;2:e111. doi: 10.1017/ash.2022.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Competency Framework for Health Workers’ Education and Training on Antimicrobial Resistance. [(accessed on 12 December 2022)]. Available online: https://www.who.int/publications/i/item/who-competency-framework-for-health-workers%E2%80%99-education-and-training-on-antimicrobial-resistance.
- 15.United Nations Sustainable Development Goals. [(accessed on 14 July 2022)]. Available online: https://sdgs.un.org/goals.
- 16.Hamilton D., Bugg I. Improving antimicrobial stewardship in the outpatient department of a district general hospital in Sierra Leone. BMJ Open Qual. 2018;7:e000495. doi: 10.1136/bmjoq-2018-000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakoh S., Yi L., Sevalie S., Guo X., Adekanmbi O., Smalle I.O., Williams N., Barrie U., Koroma C., Zhao Y., et al. Incidence and risk factors of surgical site infections and related antibiotic resistance in Freetown, Sierra Leone: A prospective cohort study. Antimicrob. Resist. Infect. Control. 2022;11:39. doi: 10.1186/s13756-022-01078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakoh S., Yi L., Russell J.B.W., Zhang J., Sevalie S., Zhao Y., Kanu J.S., Liu P., Conteh S.K., Williams C.E.E., et al. The burden of surgical site infections and related antibiotic resistance in two geographic regions of Sierra Leone: A prospective study. Ther. Adv. Infect. Dis. 2022;9:20499361221135128. doi: 10.1177/20499361221135128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakoh S., Li L., Sevalie S., Guo X., Adekanmbi O., Yang G., Adebayo O., Yi L., Coker J.M., Wang S., et al. Antibiotic resistance in patients with clinical features of healthcare-associated infections in an urban tertiary hospital in Sierra Leone: A cross-sectional study. Antimicrob. Resist. Infect. Control. 2020;9:38. doi: 10.1186/s13756-020-0701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakoh S., Maruta A., Kallon C., Deen G.F., Russell J.B.W., Fofanah B.D., Kamara I.F., Kanu J.S., Kamara D., Molleh B., et al. How Well Are Hand Hygiene Practices and Promotion Implemented in Sierra Leone? A Cross-Sectional Study in 13 Public Hospitals. Int. J. Environ. Res. Public Health. 2022;19:3787. doi: 10.3390/ijerph19073787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fofanah B.D., Abrahamyan A., Maruta A., Kallon C., Thekkur P., Kamara I.F., Njuguna C.K., Squire J.S., Kanu J.S., Bah A.J., et al. Achieving Minimum Standards for Infection Prevention and Control in Sierra Leone: Urgent Need for a Quantum Leap in Progress in the COVID-19 Era! Int. J. Environ. Res. Public Health. 2022;19:5642. doi: 10.3390/ijerph19095642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamara I.F., Kumar A.M.V., Maruta A., Fofanah B.D., Njuguna C.K., Shongwe S., Moses F., Tengbe S.M., Kanu J.S., Lakoh S., et al. Antibiotic Use in Suspected and Confirmed COVID-19 Patients Admitted to Health Facilities in Sierra Leone in 2020–2021: Practice Does Not Follow Policy. Int. J. Environ. Res. Public Health. 2022;19:4005. doi: 10.3390/ijerph19074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendelson M., Morris A.M., Thursky K., Pulcini C. How to start an antimicrobial stewardship programme in a hospital. Clin. Microbiol. Infect. 2020;26:447–453. doi: 10.1016/j.cmi.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization . Infection Prevention and Control: Report by the Director-General. WHO; Geneva, Switzerland: 2022. [Google Scholar]
- 25.Kirchhelle C., Atkinson P., Broom A., Chuengsatiansup K., Ferreira J.P., Fortané N., Frost I., Gradmann C., Hinchliffe S., Hoffman S.J., et al. Setting the standard: Multidisciplinary hallmarks for structural, equitable and tracked antibiotic policy. BMJ Glob. Health. 2020;5:e003091. doi: 10.1136/bmjgh-2020-003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Browne A.J., Chipeta M.G., Haines-Woodhouse G., Kumaran E.P.A., Hamadani B.H.K., Zaraa S., Henry N.J., Deshpande A., Reiner R.C., Jr., Day N.P.J., et al. Global antibiotic consumption and usage in humans, 2000–2018: A spatial modelling study. Lancet Planet Health. 2021;5:e893–e904. doi: 10.1016/S2542-5196(21)00280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon J., Manyau S., Kandiye F., Kranzer K., Chandler C.I.R. Antibiotics, rational drug use and the architecture of global health in Zimbabwe. Soc. Sci. Med. 2021;272:113594. doi: 10.1016/j.socscimed.2020.113594. [DOI] [PubMed] [Google Scholar]
- 28.Statistics Sierra Leone Sierra Leone Population Census Report. 2015. [(accessed on 14 January 2022)]. Available online: https://www.statistics.sl/images/StatisticsSL/Documents/Census/2015/sl_2015_phc_thematic_report_on_pop_structure_and_pop_distribution.pdf.
- 29.Health Ministry Human Resources for Health Strategy. [(accessed on 17 July 2022)];2021 Available online: https://www.afro.who.int/sites/default/files/2017-05/hrhstrategy2017.pdf.
- 30.Lakoh S., Jiba D.F., Kanu J.E., Poveda E., Salgado-Barreira A., Sahr F., Sesay M., Deen G.F., Sesay T., Gashau W., et al. Causes of hospitalization and predictors of HIV-associated mortality at the main referral hospital in Sierra Leone: A prospective study. BMC Public Health. 2019;19:1320. doi: 10.1186/s12889-019-7614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization WHO Access, Watch, Reserve, Classification of Antibiotics for Evaluation and Monitoring of Use. 2021 AWaRe Classification. [(accessed on 3 December 2021)];2021 Available online: https://www.who.int/publications/i/item/2021-aware-classification.
- 32.World Health Organization . World Health Organization Model List of Essential Medicines, 22nd List. World Health Organization; Geneva, Switzerland: 2021. [(accessed on 2 December 2021)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/345533/WHO-MHP-HPS-EML-2021.02-eng.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available at the University of Sierra Leone repository and will be available on request.