Abstract
Apolipoprotein B mRNA-editing enzyme, catalytic polypeptides (APOBECs) are cytosine deaminases involved in innate and adaptive immunity. However, some APOBEC family members can also deaminate host genomes to generate oncogenic mutations. The resulting mutations, primarily signatures 2 and 13, occur in many tumor types and are among the most common mutational signatures in cancer. This review summarizes the current evidence implicating APOBEC3s as major mutators and outlines the exogenous and endogenous triggers of APOBEC3 expression and mutational activity. The review also discusses how APOBEC3-mediated mutagenesis impacts tumor evolution through both mutagenic and non-mutagenic pathways, including by inducing driver mutations and modulating the tumor immune microenvironment. Moving from molecular biology to clinical outcomes, the review concludes by summarizing the divergent prognostic significance of APOBEC3s across cancer types and their therapeutic potential in the current and future clinical landscapes.
Keywords: APOBECs, Cancer, Somatic mutations, Germline genetics, Tumor microenvironment, Biomarkers, Immunotherapy
Background
Apolipoprotein B mRNA-editing enzyme, catalytic polypeptides (APOBECs) are a class of cytosine deaminases with eleven primary family members: APOBEC1, Activation-Induced Deaminase (AID), APOBEC2, APOBEC3 (A–H), and APOBEC4. Alternative splicing of APOBEC3B, APOBEC3H, and APOBEC3F further diversifies the APOBEC superfamily [1–4]. While all APOBEC family members share a conserved catalytic domain, they have distinct functions, mutational substrates, and tissue expression patterns [5]. AID, for example, is expressed in activated B cells and facilitates antibody diversification by deaminating immunoglobulin genes [6]. In contrast, APOBEC1 is expressed in the small intestine and edits mRNA to enable tissue-specific expression of a truncated but functionally important gastrointestinal protein [7–9]. APOBEC3s are much more widely expressed across human tissues and deaminate—and thereby damage—viral genomes as part of the innate immune response [10].
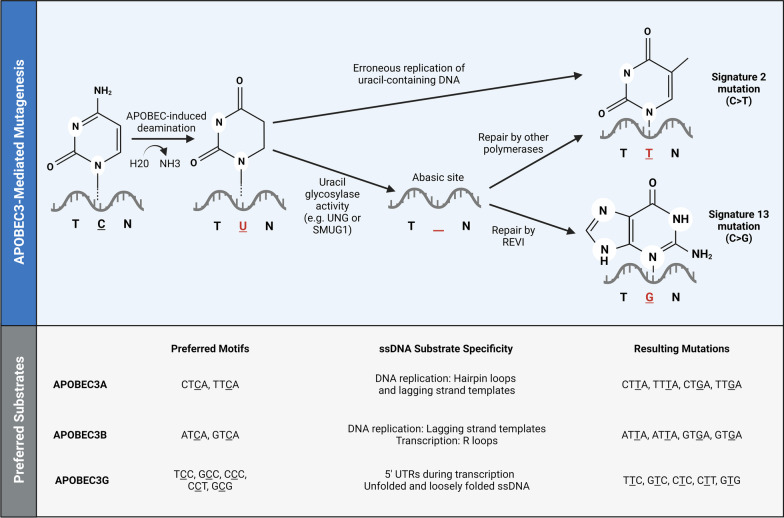
Although APOBEC3s protect cells from viral infection, they also make host DNA vulnerable to mutations. APOBEC3-mediated mutagenesis begins with cytosine deamination, and all APOBEC3s can deaminate single-stranded DNA (ssDNA) with varying levels of enzymatic activity [11–13]. ssDNA substrates for APOBEC3s can transiently arise in the double-stranded genome during normal cellular processes such as DNA replication, transcription, and genomic repair. For example, both APOBEC3A and APOBEC3B can deaminate lagging strand templates during DNA replication [14–16]. APOBEC3A can also act on hairpin loops that form during DNA replication, while APOBEC3B preferentially deaminates R loops during transcription [17, 18]. APOBEC3G may similarly act on ssDNA during transcription, especially within 5′ UTRs [15]. Additionally, APOBEC3G has been shown to deaminate unfolded and loosely folded ssDNA (Fig. 1) [19].
Fig. 1.
Mechanisms and preferred substrates for APOBEC3-mediated mutagenesis. Upper panel: APOBEC3s deaminate ssDNA, leaving uracil in the DNA template. Erroneous replication and repair pathways can then generate mutational signatures 2 and 13. Repair by the translesion synthesis (TLS) polymerase REVI generates a C-to-G mutation (signature 13), while repair by other enzymes such as DNA polymerase δ, DNA polymerase ε, and TLS polymerase κ generates a C-to-T mutation (signature 2) [20]. Lower panel: The major mutators among the APOBEC3 superfamily have distinct substrate preferences defined mainly by trinucleotide context and ssDNA secondary structure
Within ssDNA, the various APOEBC3s deaminate cytosines in distinct trinucleotide contexts. For example, APOBEC3A and APOBEC3B—which are the major mutators—deaminate thiamine preceding cytosine (TpC) motifs; APOBEC3A preferentially acts on TpC motifs following pyrimidines, while APOBEC3B tends to deaminate TpC motifs after purines [20–23]. Following deamination, different cellular processes can create C-to-T and C-to-G mutations, which are defined as signatures 2 and 13 in COSMIC, respectively [24–26]. The former C-to-T transition is more common and arises from aberrant replication of uracil-containing DNA templates, while both substitutions can occur through erroneous repair of abasic sites generated by uracil glycosylase activity (Fig. 1) [20, 27–29]. In addition to these conventionally defined APOBEC3-induced mutational signatures, APOBEC3G can cause C-to-T transitions at TCC, GCC, CCC, CCT, and GCG motifs (Fig. 1) [30].
APOBEC3-induced mutations are ubiquitous in cancer and may occur dispersed throughout the genome or in clusters. Over 75% of kataegis in tumor genomes have been attributed to APOBEC3 activity, compared to 15% of more diffuse omikli hypermutation [31, 32]. Overall, APOBEC3-induced mutations can constitute up to 68% of tumor mutation burden and are found in over half of all tumors; Only age-related signatures are more common [26, 27, 33].
Many of these APOBEC3-induced alterations are highly recurrent driver mutations affecting oncogenes and tumor suppressors, and APOBEC3s can also impact disease course through non-mutagenic pathways such as immune modulation in the tumor microenvironment. APOBEC3s can promote immune-activated or immunosuppressed phenotypes, which may partially explain their varying prognostic significance across cancer types. Based on clinical associations and pre-clinical studies, APOBEC3s could be used as biomarkers and targeted for therapies. The causes and clinical implications of APOBEC3-mediated mutagenesis are thus important areas of research and the focus of this review.
Expression of APOBEC3s in cancers
APOBEC3s are expressed at low levels in many healthy tissues but are often overexpressed in tumors. Most studies have used RNA-based profiling to detect APOBEC3 expression, and protein-based analyses have been more limited (Table 1). APOBEC3B is generally expressed at higher levels than other APOBEC3 family members, and an analysis of multiple cancers detected enrichment of APOBEC3B in eight tumor types: bladder, breast, head and neck, lung adenocarcinoma, lung squamous cell carcinoma, prostate, clear cell renal, and uterine [34]. High APOBEC3B expression was also observed in cervical and skin cancers, although healthy tissue was not available for comparison [34]. Similarly, high APOBEC3B levels have been reported in bladder, bile duct, lung, gastric, esophageal, neuroendocrine, and ovarian tumors [35–43].
Table 1.
Overexpression and correlation of APOBEC3s with mutation burden across cancers
| Cancer type | APOBEC3(s) overexpressed* | Expression type* | Correlated tumor mutation burden(s)** | References |
|---|---|---|---|---|
| Multiple | 3A, 3B*, 3F, and 3G | mRNA* | APOBEC3-induced | [27] |
| Adrenal | 3B | mRNA* and protein | Overall | [44] |
| B-cell lymphoma | 3B and 3C | mRNA | N/A | [45] |
| Bile duct | 3A* and 3B (No healthy tissue control) | mRNA | APOBEC3-induced | [43] |
| Bladder | 3B* | Protein | N/A | [35] |
| 3B | mRNA | Not detected | [27] | |
| 3B | mRNA | N/A | [34] | |
| 3A* and 3B* | mRNA* | APOBEC3-induced | [1, 46] | |
| 3B1 | mRNA and protein | N/A | [1] | |
| Breast | 3A*, 3B*, and 3H | mRNA* | APOBEC3-induced | [27] |
| 3B | mRNA | N/A | [34] | |
| 3B* | mRNA* | APOBEC3-induced, overall | [22] | |
| 3A* and 3B | mRNA* | APOBEC-induced | [47] | |
| 3A*, 3B*, and C3-H | mRNA* | APOBEC3-induced, overall | [48] | |
| Cervical | 3B | mRNA | N/A | [34] |
| Esophageal | 3B* | mRNA and protein* | APOBEC3-induced in PIK3CA | [39] |
| Gastric | 3B* | Protein* | Overall | [37] |
| 3B | mRNA and protein | N/A | [38] | |
| Glioma | 3B, 3C, 3D, 3F, 3G, 3H | mRNA* | Overall | [51] |
| Head and neck | 3B | mRNA | N/A | [34] |
| Kidney | 3B | mRNA | N/A | [34] |
| Leukemia | 3A | mRNA | N/A | [52] |
| Lung | 3B* | mRNA* | APOBEC3-induced | [27] |
| 3B* | mRNA* | APOBEC3-induced, overall | [53] | |
| 3B* | mRNA* | APOBEC3-induced | [54] | |
| 3B | mRNA | N/A | [34] | |
| 3B | mRNA | N/A | [36] | |
| Nasopharyngeal | 3B | Protein | N/A | [55] |
| Ovarian | 3B | Protein | N/A | [42] |
| 3B | mRNA* and protein | Overall | [41] | |
| 3B* | mRNA* | Overall | [56] | |
| Pancreatic | 3G | mRNA and protein | N/A | [49] |
| Penile | 3A (In subset) | Protein | N/A | [57] |
| Prostate | 3B | mRNA | N/A | [34] |
| Skin | 3B | mRNA | N/A | [34] |
| Uterus | 3B | mRNA | N/A | [34] |
*Indicates that a correlation was detected between the specified APOBEC3(s) and mutation burden(s). Some papers with both protein and mRNA expression only tested for correlations with one expression metric, so lack of an asterisk may indicate no relevant data rather than lack of statistical association
**N/A indicates no relevant data, not lack of statistical association
The expression of other APOBEC3s may also be dysregulated in cancer. APOBEC3G, for example, has been found at high levels in colon and pancreatic tumors [49, 50]. In breast cancer, studies have detected enrichment of APOBEC3A, APOBEC3B, and APOBEC3H [27, 48]. High APOBEC3 expression has also been observed in multiple hematologic cancers. For example, APOBEC3A enrichment has been noted in leukemia, and both APOBEC3B and APOBEC3C have been found at elevated levels in primary effusion lymphoma [45, 52].
APOBEC3A and APOBEC3B as the major mutators
APOBEC3 overexpression likely promotes mutagenesis, as multiple studies have identified correlations between APOBEC3 expression and a signature-specific mutation burden (Table 1). In a combined analysis of multiple tumor types, APOBEC3B expression was strongly associated with a higher APOBEC3-induced mutation load; APOBEC3A, APOBEC3F, and APOBEC3G showed similar but weaker correlations [27]. High APOBEC3B expression was also associated with more APOBEC3-induced mutations in lung cancer, while both APOBEC3A and APOBEC3B levels were correlated with APOBEC3-induced mutations in breast cancer [22, 47, 53, 54]. Similar associations have been observed in bladder cancer, which has one of the highest APOBEC3-induced mutation burden [1, 27, 34, 46]. In cholangiocarcinoma, only APOBEC3A expression was associated with APOBEC3-induced mutation burden [43].
These correlations suggest that APOBEC3A and APOBEC3B both contribute to mutagenesis, but the relative importance of these family members remains controversial [47, 58]. APOBEC3B is often assumed to be the major mutator given its higher expression in many tumors [22, 26, 34–36, 45, 48, 59]. However, APOBEC3A has greater enzymatic activity, which may allow it to generate more mutations despite generally lower tissue expression [47, 60]. Accordingly, comparison of APOBEC3 knockout cell lines found that APOBEC3A deficiency has largest effect on mutagenesis [58]. This result corroborates earlier findings in yeast, which first distinguished APOBEC3A- and APOBEC3B-induced mutations and found that the former are more abundant in tumor genomes [23]. Further implicating APOBEC3A as a predominant mutator, an APOBEC3B germline deletion that generates a chimera of the APOBEC3A coding region fused to the 5′ UTR of APOBEC3B has been associated with more APOBEC-induced mutations in some cancers [61–64].
Other APOBEC3 family members likely induce mutations, as an in vitro analysis detected continued—though significantly decreased—acquisition of signatures 2 and 13 despite knocking out both APOBEC3A and APOBEC3B [58]. APOBEC3H may contribute to this residual mutagenesis, especially in cancers with APOBEC3H haplotype I, which has strong enzymatic activity and increased nuclear localization [65]. APOBEC3G could also be mutagenic since its expression has been associated with a distinct mutational signature [30]. Multiple APOBEC3s may thus induce mutations in cancer, with the most significant mutators potentially varying across tumors.
Exogenous and endogenous triggers of APOBEC3-mediated mutagenesis
Viral infection
APOBEC3s are interferon-stimulated genes induced by a wide variety of viruses, including polyomaviruses, parvoviruses, herpesviruses, and hepatitis B viruses [66]. Many virus-associated cancers thus have a high loads of mutation signatures 2 and 13. Cervical cancer, for example, is caused by human papillomavirus (HPV) in over 95% of cases and has abundant APOBEC3-induced mutations [67, 68]. APOBEC-induced mutations are also common in head and neck squamous cell carcinoma (HNSCC), with a strong correlation between HPV positivity and mutation signatures 2 and 13 [69].
Viral infection may also contribute to APOBEC3-mediated mutagenesis in some cancers that are not traditionally understood as virus-associated. According to the “hit and run” hypothesis, viral infection can trigger APOBEC3 activity early in tumorigenesis but is cleared before tumor detection [70]. This postulation could be relevant for some cases of bladder cancer, as a history of BK polyomavirus (BKPyV)-positive urine has been associated with increased bladder cancer risk [71]. BKPyV infection was also shown to induce APOBEC3 expression and deamination activity in an in vitro model of the normal human urothelium [72]. The potential risk of BKPyV-induced bladder carcinogenesis may be especially high in immunocompromised populations, specifically in organ transplant recipients. Accordingly, BKPyV viremia or other polyomavirus-related complications have been associated with a four-fold higher risk of bladder cancer following kidney transplantation [73–77]. Deep sequencing of bladder tumor genomes from organ transplant recipients has also revealed BKPyV integration [78–80].
While certain viral infections may contribute to APOBEC3-mediated mutagenesis in some traditionally non-viral cancers, additional factors are likely important given the continued acquisition of APOBEC3-induced mutations late in tumor evolution and presumably after infection clearance [22, 56, 81, 82]. Such non-viral factors may also explain the prevalence of APOBEC3-induced mutations in cancers for which hit-and-run viral etiology is less plausible.
Inflammation
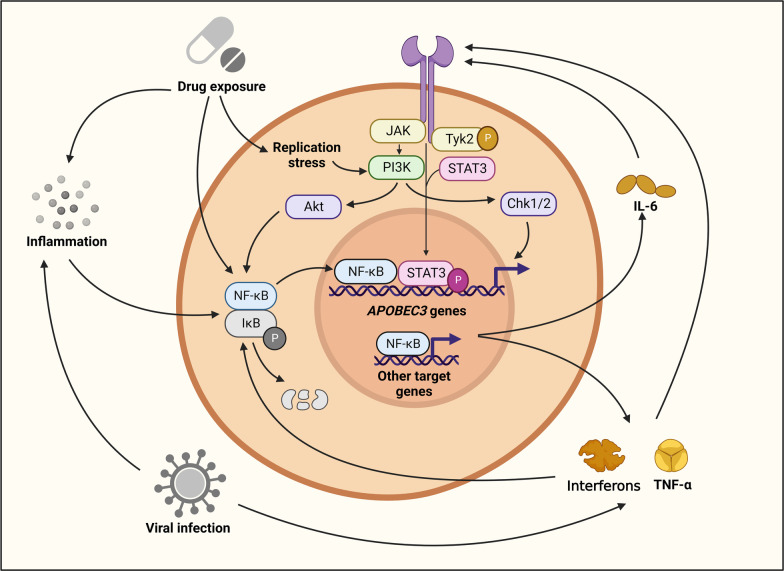
A myriad of factors can induce inflammation, which may increase APOBEC3 expression via NF-κB signaling—a major inflammatory pathway. Supporting this hypothesis, three NF-κB binding sites have been detected in the APOBEC3B promoter, and p65/p50 and p65/c-Rel heterodimers—which are part of the canonical NF-κB pathway—have proved important for APOBEC3B transcription [83]. Non-canonical NF-κB signaling may also regulate APOBEC3 expression, as the APOBEC3B promoter contains a RelB binding site. Multiple known APOBEC3 inducers such as LPS and IL-4 are also potent NF-κB activators, which further implicates NF-κB as a transcriptional driver of APOBEC3s during inflammation [84].
NF-κB signaling may also increase APOBEC3 expression indirectly via the transcription of pro-inflammatory mediators. For example, the NF-κB target gene IL-6 has been shown to induce APOBEC3B expression in hepatocellular carcinoma via JAK/STAT signaling [85, 86]. Similarly, TNF-α has been found to promote APOBEC3A expression in keratinocytes [87, 88]. Corroborating these findings, a study of cholangiocarcinoma and gallbladder cancer found upregulation of both APOEBC3A and APOBEC3B with IL-6 and TNF-α exposure [43]. Additionally, IFN-γ has been implicated as a driver of APOBEC3B expression in bladder tumors and lung adenocarcinoma [46, 89]. NF-κB signaling—with the ability to act both directly through APOBEC3 transcription and indirectly through other inflammatory mediators—is thus a likely driver of APOBEC3-mediated mutagenesis in cancer (Fig. 2). This APOBEC3-inducing role of NF-κB may be especially important in immunologically “hot” tumors with highly inflamed microenvironments.
Fig. 2.
Transcriptional regulation of APOBEC3 Genes. NF-κB signaling is a shared pathway for endogenous and exogenous APOBEC3 triggers. To induce APOBEC3s, NF-κB signaling acts directly via transcription of APOBEC3s and indirectly through the transcription of other inflammatory mediators. Key inflammatory mediators include interferons, TNF-α, and IL-6, which can drive APOBEC3 transcription through NF-κB and JAK/STAT signaling. Additionally, replication stress can activate NF-κB signaling via PI3K/Akt to promote APOBEC3 expression
Drug exposure and replication stress
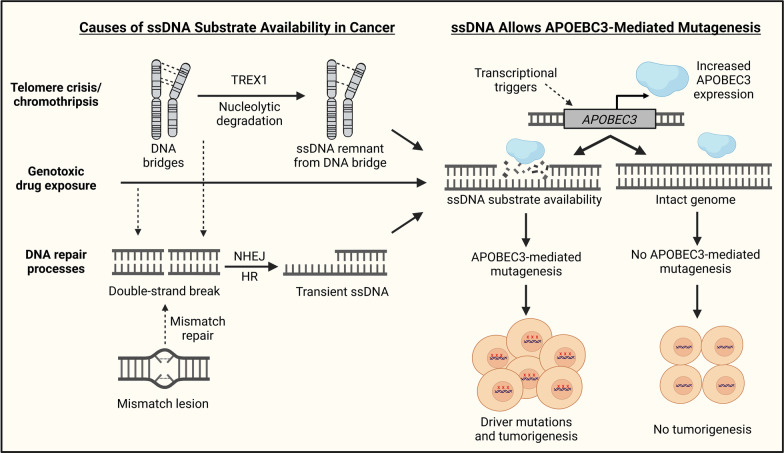
Exposure to certain drugs can also trigger APOBEC3-mediated mutagenesis, and chemotherapies such as bleomycin, cisplatin, etoposide, 5-fluorouracil, gemcitabine, hydroxyurea, aphidicolin, and camptothecin have all been shown to induce APOBEC3 expression in cancer cell lines [1, 61, 90, 91]. NF-κB signaling likely mediates APOBEC3 induction in response to some of these drugs, but replication stress and PI3K signaling may also play major role (Fig. 2) [1, 90, 92, 93]. In addition to increasing APOBEC3 expression, genotoxic drugs may further fuel APOBEC3-mediated mutagenesis by inducing genomic damage and thereby generating ssDNA substrates (Fig. 3).
Fig. 3.
Two-factor model for APOBEC3-mediated mutagenesis. In a two-factor model, APOBEC3-mediated mutagenesis needs both induction of APOBEC3 expression and availability of ssDNA. Factors such as drug exposure, telomere crisis, and DNA repair processes can generate ssDNA, while transcriptional triggers can upregulate APOBEC3 expression [94–96]
Even in the absence of genotoxic drugs, cancer cells may experience APOBEC3-inducing replication stress due to accumulated DNA damage and specific oncogenic mutations. In breast cancer, for example, PTEN depletion and HER2 amplification have been shown to induce replication stress and increase APOBEC3B activity in vitro [90]. In lung cancer, the loss of FHIT1—a common genetic alteration that causes replication stress—was associated with a higher APOBEC3-induced mutation burden [53]. These replication stress-inducing mutations may be especially strong triggers of APOBEC3-mediated mutagenesis, as they cause persistent cellular changes in contrast with the more transient APOBEC3 induction following viral infection or drug exposure. In a positive feedback loop, increased APOBEC3 expression may exacerbate replication stress due to additional DNA damage, slowed replication forks, and cell cycle arrest [52, 97–99].
In the context of replication stress, APOBEC3s—particularly APOBEC3B—may be especially prone to causing kataegis rather than more diffuse mutation patterns. Accordingly, APOBEC3B has been shown to induce kataegis in cancer cell lines during telomere crisis [94]. APOBEC3B was similarly associated with kataegis in p53-defecient cell lines, and a pan-cancer analysis found that APOBEC3B expression was positively correlated with kataegis [31, 99].
Somatic and germline alterations
Somatic mutations in multiple genes have been associated with increased APOBEC3-mediated mutagenesis, but whole-genome sequencing analyses have not detected recurrent somatic single-nucleotide variants in the coding or regulatory regions of APOBEC3s [100, 101]. Somatic mutations that increase the deamination activity of APOBEC3s are thus unlikely, as are mutations that alter APOBEC3 expression via increased promoter or enhancer activity. However, increased APOBEC3 expression may occur due to copy number amplification in cancers. Although only noted in one cancer type, APOBEC3 copy number variation was found in ~ 30% of lung tumors [36, 102]. This genetic alteration was associated with increased APOBEC3B expression and a higher APOBEC-induced mutation burden [36].
Germline variants at the APOBEC3 gene locus appear to be more common and can influence APOBEC3 expression and cancer risk. For example, the single-nucleotide polymorphism (SNP) rs1014971—which is located upstream of the APOBEC3 gene cluster—is associated with increased APOBEC3B expression, enrichment of APOBEC3-induced mutations, and higher bladder cancer risk [61, 63, 103]. In breast cancer, a deletion polymorphism that generates an APOBEC3A/B chimera is associated with increased disease risk, more APOBEC3-induced mutations, and poor tumor differentiation (a sign of negative prognosis) [47, 61, 62, 64, 104, 105]. The same deletion polymorphism may contribute to APOBEC3-induced hypermutation in acute lymphoblastic leukemia [62]. In lung cancer, the combination of six SNPs that define APOBEC3H haplotype I have been associated with increased disease risk [65] (Table 2). Genetic variation within this haplotype may further enhance lung cancer risk [106]. Additionally, the variant rs2267401 has been shown to increase gallbladder cancer and hepatocellular carcinoma risk, likely by increased APOBEC3B expression that arises through heightened promoter activity and IL-6 response [43, 86].
Table 2.
Germline variants affecting APOBEC3s and cancer risk
| Germline variant | Genomic location | Risk allele or amino acid | Cancer | Molecular impact* | Effect on cancer risk* | References |
|---|---|---|---|---|---|---|
| rs139293 | Exon 2 of APOBEC3H | T | Lung | Activity-decreasing amino acid change (R18L) and lower expression of APOBEC3H and APOBEC3C | Decreased | [107] |
|
rs1014971 Linked proxies: rs17000526 rs1004748 |
Intergenic region ~ 25 kb from APOBEC3A | T | Bladder | Increased APOBEC3B expression and more APOBEC3-induced mutations | Increased | [61] |
| N/A | Increased | [63] | ||||
| N/A | Increased overall and for high-risk tumors | [103] | ||||
| Breast | Increased APOBEC3B expression but no association with APOBEC3-induced mutation burden | Moderately increased | [61] | |||
| rs2267401 | APOBEC3B promoter | G | Bile duct | Increased APOBEC3B promoter activity | Increased | [43] |
| Liver | Increased APOBEC3B expression with stronger promoter response to IL-6 and more APOBEC3-induced HBV mutations | Increased | [86] | |||
| Gallbladder | Decreased APOBEC3B promoter activity | Decreased | [43] | |||
| rs12157810 | APOBEC3A promoter | C | Bile duct | Increased APOBEC3A promoter activity | Decreased | [43] |
| Gallbladder | Increased APOBEC3A promoter activity | Decreased | [43] | |||
| Renal | Higher APOBEC3A expression and increased promoter sensitivity to TNF-α | Decreased | [108] | |||
| Colorectal | No change in promoter responsiveness to TNF-α, but decreased response to IL-6 | N/A | [108] | |||
| APOBEC3H Haplotype I | APOBEC3H | Lung | Gly105 creates unstable APOBEC3H protein with increased nuclear localization | Increased | [65] | |
| rs34522862 | Exon 2 | Asn | ||||
| rs139293 | Exon 2 | Arg | ||||
| rs139297 | Exon 3 | Gly | ||||
| rs139299 | Exon 3 | Lys | ||||
| re139298 | Exon 3 | Lys | ||||
| rs139302 | Exon 4 | Glu | ||||
| rs139298 | APOBEC3H Exon 3 | Glu | Lung | K121E within APOBEC3H haplotype I destabilizes protein, potentially creating fewer mutations to avoid immune response | Decreased relative to standard haplotype I | [106] |
| APOBEC3A/B chimera Proxy: rs12628403 | Coding region of APOBEC3B | ~ 30 kb deletion | Breast | Enrichment of APOBEC3-induced mutations | Increased | [61] |
| Increased APOBEC3-induced mutation load | N/A | [62] | ||||
| Higher abundance of tumor-infiltrating immune cells | Increased | [109] | ||||
| N/A | Increased for higher histological grade | [64] | ||||
| Hypermutated phenotype with immune activation | N/A | [105] | ||||
| Leukemia | Hypermutated tumor genome | N/A | [62] | |||
| Ovarian | N/A | Decreased | [110] | |||
| Bladder | No association | No association | [61] | |||
| APOBEC3B indel | Exon 5 of APOBEC3B | Deletion | Breast | N/A | Increased | [111] |
*N/A indicates that the study did not test for molecular impact or clinical association
In contrast, the same SNP rs2267401 was associated with decreased risk of cholangiocarcinoma and lower APOBEC3B promoter activity in this tumor type, likely due to overexpression of the transcriptional repressor TFAP2A [43]. Another variant, rs12157810, has also been associated with lower cholangiocarcinoma and gallbladder cancer risk, although it was found to increase APOBEC3A promoter activity [43]. Concordant results were observed in renal cancer [108]. Another SNP rs139293 has been associated with decreased risk of lung cancer [107]. Located in an exon, this variant creates a potentially activity-decreasing amino acid change in APOBEC3H and may lower the expression of APOEBC3H and APOBEC3C [107] (Table 2).
Retrotransposon activity, telomere crisis, and DNA damage
Cancer cells have generally unstable genomes that can fuel APOBEC3-mediated mutagenesis through multiple distinct pathways. Specifically, genomic instability may promote retrotransposon activity and thereby trigger episodic bursts of APOBEC3-mediated mutagenesis [112]. Genomic instability is also associated with telomere crisis and chromothripsis, which have been shown to generate ssDNA breakpoints deaminated by APOBEC3B [94] (Fig. 3). APOBEC3-mediated mutagenesis can be triggered by upregulation of APOBEC3s during DNA damage-induced replication stress and associated mismatch repair processes such as non-homologous end joining (NHEJ), and homologous recombination (HR) that generate ssDNA intermediates [90, 95, 96, 113–116] (Fig. 3). Since APOBEC3s can deaminate lagging strand templates, the rapidly dividing cancer cells can have frequently exposed ssDNA and thereby fuel APOBEC3-mediated mutagenesis [14].
These endogenous processes in cancer cells may drive the continued acquisition of APOBEC3-induced mutations late in tumor evolution without exposure to exogenous triggers [81]. The importance of DNA damage and ssDNA substrate availability may also explain why APOBEC3s are expressed at low levels in many healthy tissues and do not typically cause considerable tumor genome-like mutation patterns. Significant APOBEC3-induced mutation loads have not been detected in healthy esophageal or endometrial gland tissue, though low levels of such mutations have been found in a subset of non-cancerous intestinal crypts and bronchial epithelial cells [59, 117–120]. A two-factor model for APOBEC3 activity is thus plausible in many cancers, with elevated APOBEC3 expression and pre-existing DNA damage both being required for APOBEC3-mediated mutagenesis (Fig. 3).
Smoking?
Smoking is a major risk factor for multiple cancers with high APOBEC3-induced mutation loads. For example, smoking accounts for over 50% of bladder cancer risk and is estimated to cause 80–90% of lung tumors [121, 122]. Smoking is most strongly associated with mutational signatures 4, 5, and 29, but multiple studies have tested whether tobacco exposure is also linked with the APOBEC3-induced signatures 2 and 13 [24–26, 123, 124]. However, the results have been contradictory. In an analysis of lung adenocarcinoma, signatures 2 and 13 were enriched in tumors from smokers [123]. In bladder cancer, signature 13 was enriched in tumors from former smokers [125]. However, a separate analysis of only muscle-invasive bladder tumors found that signature 13 was enriched in non-smokers and negatively correlated with signature 5 [124]. Additionally, an in vitro study in normal human urothelium found that that exposure to benzo[a]pyrene—a procarcinogen in cigarette smoke—did not induce signature 2 or13. However, this study has limitations in that it only used one procarcinogen, while tobacco smoke includes approximately 60 carcinogens [126].
If smoking does increase APOBEC3-mediated mutagenesis, the effect is likely due to generalized DNA damage that increases ssDNA substrate availability [123]. Although the nicotine component of tobacco can induce inflammation and NF-kB signaling, there is no evidence that tobacco smoke directly increases APOBEC3 expression. A single study of non-small cell lung cancer tested this hypothesis but found no association between smoking and APOBEC3B mRNA expression [54]. However, the prevalence of smoking as a common risk factor for cancers where APOBEC3-mediated mutagenesis is widely operational might make it hard to decern any interactions between smoking and APOBEC3s.
Impact of APOBEC3s through mutagenic and non-mutagenic pathways
APOBEC3-induced oncogenic coding mutations
APOBEC3-induced mutations are ubiquitous in cancer and can drive carcinogenesis by activating oncogenes or inactivating tumor suppressors (Table 3). For example, the APOBEC3-induced FGFR3 S249C mutation—the most common FGFR3 mutation in bladder cancer—causes constitutive activation of the encoded growth factor receptor to promote cell proliferation [127, 128]. Although less recurrent in other tumor types, S249C has also been detected in lung, cervical, and head and neck cancers [129–132]. In addition to activating receptors, APOBEC3-induced mutations can affect downstream signaling pathways to generate highly oncogenic mutational synergies. The APOBEC3-induced PIK3CA E545K mutation, for example, causes aberrant activation of the growth-promoting PI3K pathway and has been detected in bladder, breast, cervical, colorectal, esophageal, head and neck, and lung tumors [39, 132–137]. The highly similar PIK3CA E542K mutation has been found in bladder, breast, cervical, colorectal, esophageal, head and neck, and lung cancers [39, 132, 133, 135, 137–140].
Table 3.
Recurrent APOBEC3-induced somatic mutations and copy number alterations in cancer
| Mutation type | Gene name | Gene type | Amino acid alteration | Cancer type(s) | References |
|---|---|---|---|---|---|
| Coding | EGFR* | Oncogene | T790M | Lung | [82, 141] |
| FGFR3 | Oncogene | S249C | Bladder, cervical, HNSCC, and lung | [127, 129–132] | |
| MEK2 | Oncogene | L46F | Melanoma | [82, 142] | |
| PIK3CA | Oncogene | E545K | Bladder, breast, cervical, colorectal, esophageal, HNSCC, and lung | [39, 132–137] | |
| PIK3CA | Oncogene | E542K | Bladder, breast, cervical, colorectal, esophageal, HNSCC, and lung | [39, 132, 133, 135, 137–140] | |
| FBXW7 | Tumor suppressor | R505G | HNSCC, upper digestive tract, urinary tract, and lung | [132, 143] | |
| Non-coding regulatory | ADGR6/GPR126 | Oncogene | N/A | Bladder | [33, 144, 145] |
| TBC1D12 | Oncogene | Bladder and breast | [144, 147] | ||
| LMO1 | Oncogene | T-Cell acute lymphoblastic leukemia | [146] | ||
| PLEKHS1 | Oncogene | Bladder and breast | [144, 147] | ||
| WDR74** | Oncogene | Bladder and breast | [144, 147] | ||
| LEPROTL1 | Unknown | Bladder and breast | [144, 155] | ||
| Copy number amplification | AGAP2 | Oncogene | N/A | Glioma | [51] |
| CDK4 | Oncogene | ||||
| EGFR | Oncogene | ||||
| MAPKAPK2 | Oncogene | ||||
| USP15 | Oncogene | ||||
| Not specified*** | EGFR | Oncogene | N/A | Bladder, breast, HNSCC, and lung | [148] |
| PTEN | Tumor suppressor | ||||
| TP53 | Tumor suppressor |
*C-to-T mutation that is not in the preferred APOBEC3 motif but has been hypothesized to be a rare APOBEC3-induced mutation that is highly selected for given its strong oncogenic effects
**Mutation has a less clear association with APOBEC3s and may arise from other mutational processes
***Subclonal mutations in cancer driver genes, but the mutations are not specified as coding or non-coding
APOBEC3-induced and other oncogene-activating mutations may allow cancer cells to proliferate unchecked in the presence of additional mutations in tumor suppressor genes—some of which arise from APOBEC3-mediated mutagenesis. For example, the inactivating R505G FBXW7 mutation is attributable to APOBEC3s and has been detected in HNSCC, upper digestive tract cancers, urinary tract cancers, and lung cancers [132, 143]. Subclonal APOBEC3-induced mutations have also been observed in the tumor suppressor genes PTEN and TP53 in bladder, breast, head and neck, and lung tumors [148].
Other mutational processes can also inactivate tumor suppressors, and high APOBEC3 activity may generate a selective pressure favoring such mutations. APOBEC3s create a high overall tumor mutation burden, so cells with impaired DNA damage response (DDR) due to tumor suppressor mutations are more likely to avert apoptosis and continue proliferating. Accordingly, TP53 mutations—which are mostly non-APOBEC3-induced—were more common in bladder cancers, lung adenocarcinomas, and B-cell lymphoma cell lines with high burden of APOBEC3-induced mutations [46, 89, 149]. Similarly, high APOBEC3B expression has been associated with more p53 mutations in breast cancer and adrenocortical carcinoma [22, 44]. In addition to selection pressure, this trend could arise from higher APOBEC3 expression in p53-mutated tumors since p53 may suppress APOBEC3B transcription via p21 and DREAM proteins [150].
Recurrent APOBEC3-induced non-coding mutations
Some APOBEC3-induced mutations in non-coding regulatory regions are also highly recurrent and may contribute to tumor development by modulating the expression of cancer-related genes (Table 3). For example, many bladder and breast tumors harbor APOBEC3-induced “twin mutation hotspots” in the promoters of PLEKHS1 and TBC1D12, which are potential oncogenes associated with invasive disease and poor prognosis [33, 144, 147, 151, 152]. APOBEC3-induced ADGR6/GPR126 enhancer mutations implicated in angiogenesis have also been detected in bladder cancer [33, 145]. Other genes with non-coding mutations attributable to APOBEC3s in bladder cancer include LEPROTL1 and potentially the tumor suppressor WDR74 [144, 153, 154]. Additionally, many T-cell acute lymphoblastic leukemias harbor an APOBEC3-induced mutation upstream of the transcription start site for the oncogene LMO1 [146]. Several such mutations were found functional and having potential oncogenic role in breast cancer [155]. However, what triggers APOBEC3-mediated mutagenesis in non-coding intergenic and promoter regions has not been decerned, but it likely occurs during DNA replication, and transcription initiation.
In addition to generating point mutations in regulatory regions, APOBEC3s may further dysregulate gene expression by promoting increased oncogene copy number (Table 3). For example, higher APOBEC3 expression has been associated with increased copy number variation of multiple Ras/MAPK regulatory genes such as ATP2B4, MAPKAPK2, and USP15 in glioma [51]. Amplification of EGFR and CDK4—both known oncogenes—has also been observed in APOBEC3-high gliomas [51]. The mechanisms through which APOBEC3s facilitate copy number changes remain unknown. Though it is plausible that APOEBC3-induced kataegis promotes chromosomal instability and double strand breaks, creating opportunities for copy number changes [156, 157].
Tumor evolution
Although some APOBEC3-induced mutations are likely important for initial tumor formation, many occur later in tumor evolution. Supporting this paradigm, episodic bursts of APOBEC3-mediated mutagenesis were observed during prolonged culture of numerous cancer cell lines [81, 112]. This repeated APOBEC3 activity can create a high overall tumor mutation burden and fuel subclone heterogeneity in a tumor context. Accordingly, APOBEC3s have been identified as primary drivers of subclonal mutations in bladder, breast, head and neck, and lung cancers [48, 148]. In bladder cancer, over 45% of subclonal mutations in driver genes may be attributable to APOBEC3s [148]. Additionally, APOBEC3-induced mutation load has been strongly associated with tumor heterogeneity in metastatic thoracic cancers [89]. APOBEC3-induced tumor heterogeneity can promote resistance to cancer therapies. While the continued acquisition of signatures 2 and 13 is often part of natural tumor evolution, chemotherapy treatment may further fuel APOBEC3-mediated mutagenesis by triggering APOBEC3 expression and inducing DNA damage [1, 81, 90, 91].
Specific therapy resistance mutations can also arise due to APOBEC3 activity. For example, the APOEBC-induced MEK2 L46F mutation may confer resistance to BRAF inhibitors such as vemurafenib and dabrafenib in melanoma [82, 142]. In lung cancer, the potentially APOBEC3-induced C > T EGFR T790 mutation can promote resistance to the EGFR inhibitors gefitinib and erlotinib [82, 141]. Similarly, some APOEBC3-induced mutations observed in relapsed refractory multiple myeloma may contribute to acquire therapy resistance [158].
Non-mutagenic pathways
APOBEC3-induced mutations are significant drivers of tumorigenesis, but APOBEC3s can also play role in cancer through non-mutagenic pathways. Exemplifying the importance of these pathways, a study of hepatocellular carcinoma found that overexpression of catalytically inactive APOBEC3B increased cell proliferation, cell migration, and cell invasion in vitro [159]. In the presence of K-Ras mutations, APOEBC3A may also promote STING-dependent metastasis and chromosomal instability based on a mouse model of pancreatic ductal adenocarcinoma [157].
Overexpression of catalytically inactive APOBEC3B has also been associated with more frequent “G1 escape,” suggesting that APOBEC3B contributes to cell cycle dysregulation [159]. Similar APOBEC3B-mediated cell cycle progression has been observed in bladder cancer [159, 160]. APOBEC3s can also inhibit cell death via multiple mechanisms: APOBEC3G was shown to inhibit anoikis via Akt activation in pancreatic cancer, and APOBEC3B may decrease cell death in gastric cancer by inhibiting PDCD2 function and lowering ATM and Chk1/2 activity [38, 49].
Additionally, APOBEC3s may affect the expression of oncogenes and tumor suppressors through epigenetic-coupled mechanisms. For example, APOBEC3B has been shown to drive estrogen receptor (ER) overexpression in breast cancer through transient chromatin remodeling [161]. Providing further evidence of APOBEC3-mediated epigenetic regulation, high APOBEC3B expression has been associated with greater LINE1 methylation (a proxy for global DNA methylation) in esophageal cancer [39].
Modulation of the tumor immune microenvironment
APOBEC3s can also impact tumor growth through non-mutagenic pathways that shape the tumor immune microenvironment (Table 4). APOBEC3s are immunosuppressive in some cancers, and higher APOBEC3B expression has been associated with less immune cell infiltration in adrenocortical carcinoma and gastric cancer [37, 162]. APOBEC3 expression has also been associated with greater infiltration of immunosuppressive mediators such as myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) in a mouse model of hepatocellular carcinoma [163].
Table 4.
Immune-modulating effects of APOBEC3s by cancer type
| Cancer type | APOBEC3(s)* | Effect on immune system | Immune features associated with APOBEC3s | Study type | References |
|---|---|---|---|---|---|
| Adrenal | 3B | Suppressive | Decreased immune cell infiltration | Patient | [162] |
| Gastric | 3B | Suppressive | Fewer CD8+ T cells expressing effector antigens and immune checkpoint proteins | Patient | [37] |
| Liver | 3B | Suppressive | More immunosuppressive mediators such as MDSCs and TAMs | Mouse | [163] |
| Breast | 3B | Activating | More CD4+ and CD8+ T cells with increased IFN-γ and cytotoxic granzyme B production. Fewer T-reg, Th2, and TAM cells | Mouse | [164] |
| 3B | Activating | More tumor-infiltrating lymphocytes | Patient | [165] | |
| 3B | Activating | Immune activation in pan-cancer analysis | Patient | [162] | |
| 3C-H | Activating | More CD8+ T-cells with greater receptor diversity and cytolytic activity | Patient | [48] | |
| Bladder | 3B | Activating | Enrichment of immune-related signatures, including IFN-γ | Patient | [46] |
| Mutation burden | Activating | Higher neoantigen loads, activated CD4+ T cells, CD8+ T cells, and M1 cells. Fewer MDSCs and naïve CD4+ T cells | Patient | [160] | |
| 3B | Activating | More cytotoxic T-cells with a higher CD8+/CD3+ ratio | Patient | [35] | |
| Glioma | 3B, 3C, 3D, 3F, 3G, 3H | Activating | Increased myeloid cell infiltration, IFN-γ signaling, and PD-L1/2 expression | Patient | [51] |
| Lung | 3B | Activating | More activated CD4+ T cells, CD8+ T cells, and NK cells. Fewer regulatory T cells | Patient | [166] |
| 3B | Activating | Upregulation of immune genes and more T cell infiltration to the tumor site | Patient | [36] | |
| Mutation burden | Activating | Enrichment of interferon pathways | Patient | [89] | |
| Melanoma | 3B | Activating | Immune activation in a multi-cancer analysis | Patient | [162] |
| Ovarian | 3B | Activating | Greater tumor immune cell infiltration | Patient | [41] |
| 3G | Activating | Higher immune cell counts in the tumor microenvironment | Patient | [167] |
*Where applicable, “mutation burden” refers to APOBEC3-induced mutation burden
The opposite effect has been observed in other cancer types, with APOBEC3s promoting immune activation. In a pan-cancer analysis, high APOBEC3B expression was associated with increased immune activation in cutaneous melanoma and breast cancer [162]. Additional studies in breast cancer have found similar results. High APOBEC3B expression has been associated with more tumor infiltrating lymphocytes, and APOBEC3B induction drove a robust T cell-mediated immune response in a mouse model [164, 165]. APOBEC3C-H levels were also correlated with more CD8+ T cells in the tumor microenvironment, increased T cell receptor diversity, and greater cytolytic activity [48, 64].
Similar immune activation has been observed in bladder cancer, with multiple studies detecting increased immune signatures and interferon signaling in APOBEC3-high tumors [35, 46, 160]. In lung cancer, T cell-mediated immune activation was linked with high APOBEC3B expression or high APOBEC3-induced mutation loads [36, 166]. In ovarian cancer, elevated APOBEC3B and APOBEC3G expression have been associated with greater immune cell infiltration [41, 167]. Although not in the tumor itself, increased APOBEC3A expression has been shown to shift macrophage polarization to a pro-inflammatory, immune-activating state [168].
Clinical and therapeutic significance of APOBEC3s in cancers
Association with poor prognosis in multiple cancers
Immunosuppression may synergize with APOBEC3-mediated mutagenesis in some cancers, allowing oncogenic mutations to accumulate and drive tumorigenesis while averting the host immune response. In accordance with this model, studies in adrenocortical carcinoma and gastric cancer—which both show immunosuppression with APOBEC3s—have found that higher APOBEC3B expression is correlated with shorter survival (Tables 4 and 5) [37, 38, 44]. Elevated APOBEC3 expression has also been associated with adverse clinical outcomes in nasopharyngeal carcinoma, clear cell renal carcinoma, and neuroendocrine tumors, though little is known about APOBEC3-mediated immune effects in these cancers (Table 5) [55, 169].
Table 5.
Association of APOBEC3 expression and APOBEC3-induced mutations with clinical outcomes by cancer type
| Cancer type | APOBEC3(s)* | Outcome type | Immunotherapy context? | Clinical outcome | References |
|---|---|---|---|---|---|
| Adrenal | 3B | Adverse | No | Shorter overall and disease-free survival | [44] |
| Bladder | 3B | Positive | No | Longer survival with APOBEC3B-enhancing SNP (rs101497) | [61] |
| Mutation burden | Positive | No | Longer survival in muscle-invasive disease cohort | [170] | |
| Mutation burden | Positive | No | Longer survival | [46] | |
| 3D and 3H | Positive | No | Better overall survival | [171] | |
| 3B | Adverse | No | Shorter progression-free survival with mutagenic A3B1 isoform | [1] | |
| Mutation burden | Adverse | No | Higher risk of class 2a tumors and poor outcomes for non-muscle invasive disease | [172] | |
| 3A and 3B | Adverse | No | Increased risk of class 2a tumors | [173] | |
| Mutation burden | Positive | Yes | Longer survival overall and better response to immune checkpoint blockade therapy | [160] | |
| Mutation burden | Positive | Yes | Increased responsiveness to immunotherapy | [174] | |
| Breast | 3A | Adverse | No | Advanced tumor stage, higher histological grade, and more lymph node involvement | [64] |
| 3A | Adverse | No | Shorter overall survival | [175] | |
| 3B | Adverse | No | Higher tumor grade with lymph node involvement and worse prognosis | [105] | |
| 3B | Adverse | No | Higher nuclear grade and more frequent lymph node metastasis | [176] | |
| 3B | Adverse | No | Shorter disease-free, metastasis-free, and overall survival. Lower overall survival in ER+ subgroup | [177] | |
| 3B | Adverse | No | Shorter survival in ER+ but not ER- group | [161] | |
| 3B | Adverse | No | Lower recurrence-free survival in a combined analysis and ER+ subgroup | [178] | |
| 3A/B | Adverse | No | Higher histological grade with 3A/B chimera | [64] | |
| 3A-H | Mixed | No | Aggressive pathology and shorter survival (3A and 3B). Improved survival (3C-H) | [48] | |
| 3B | Mixed | No | Shorter replace-free, metastasis-free, and overall survival (mRNA). Increased disease-free survival (protein) | [165] | |
| 3B | Mixed | No | Higher nuclear grade and Ki-67 labeling index, but improved responsiveness to neoadjuvant chemotherapy | [179] | |
| Mutation burden | Adverse (overall) | Yes | Shorter survival with first-line endocrine and CDK4/6i therapy, but improved response to immune checkpoint blockade therapy | [180] | |
| Positive (immunotherapy) | |||||
| Mutation burden | Positive | Yes | Improved response to immunotherapy | [181] | |
| Esophageal | 3B | Adverse/none | No | Higher histological grade | [39] |
| Gastric | 3B | Adverse (overall) | No | Shorter overall and progression-free survival, but improved response to fluorouracil-based adjuvant chemotherapy and PD-1 blockade therapy | [37] |
| Positive (immunotherapy) | Yes | ||||
| 3B | Adverse | No | Aggressive pathology and poor survival | [38] | |
| Glioma | 3B, 3C, 3D, 3F, 3G, 3H | Adverse | No | Poor prognosis, especially in the low-grade subtype | [51] |
| Head and neck | Mutation burden | Positive | Yes | Improved responsiveness to immunotherapy | [174] |
| Lung | 3B | Adverse | No | Shorter survival | [182] |
| 3B | Adverse | No | Shorter disease-free and overall survival with more with lymph node involvement and higher TNM stage | [183] | |
| 3B | Adverse | No | Shorter overall survival | [175] | |
| Mutation burden | Adverse | No | Worse overall survival | [184] | |
| Mutation burden | Adverse | No | Signature 2 (but not 13) burden associated with poor prognosis | [185] | |
| 3B | None | No | No association with pathological features or clinical outcomes | [54] | |
| Mutation burden | Positive | Yes | Increased response to immune checkpoint blockade therapy | [166] | |
| 3B | Positive | Yes | Improved responsiveness to immune checkpoint blockade therapy | [36] | |
| Nasopharyngeal | 3B | Adverse | No | Aggressive disease and poor outcomes | [55] |
| Neuroendocrine tumor | 3B | Adverse | No | More lymph node metastasis | [40] |
| Ovarian | Mutation burden | Positive | No | Improved progression-free and overall survival | [186] |
| 3B | Adverse | No | Shorter disease-free and overall survival | [42] | |
| 3B | Positive | No | Longer progression-free (significant) and overall (not significant) | [41] | |
| 3G | Positive | No | Favorable clinical outcomes with A3G, but no association for A3B | [167] | |
| Pancreatic | 3G | None | No | No association with tumor stage, tumor type, or prognosis | [49] |
| Renal | 3B | Adverse | No | More frequent recurrence | [169] |
*Where applicable, “mutation burden” refers to APOBEC3-induced mutation burden
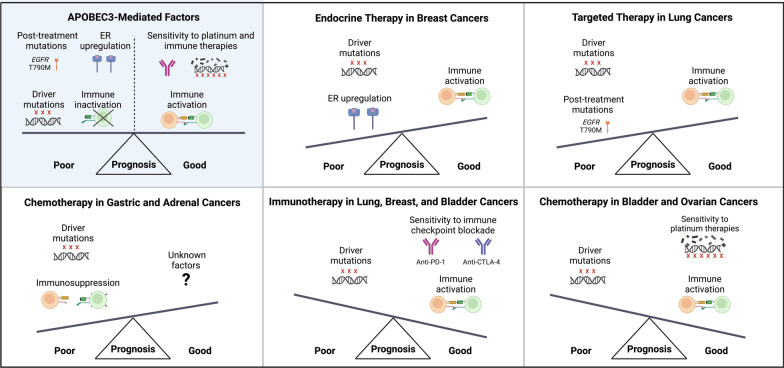
High APOBEC3 expression or induced mutation burden may also predict adverse outcomes in breast cancer, although some reports have found inconsistent results (Table 5). Since APOBEC3s induce immune activation in breast cancer, additional factors must overcome this heightened immune response to drive tumorigenesis (. 4). Greater estrogen receptor activation (ER) is a likely mechanism since APOBEC3B has been shown to promote ER overexpression in breast cancer [161]. Accordingly, associations between high APOBEC3B expression and adverse clinical outcomes are stronger in ER+ disease (Table 5).
Fig. 4.
Effects of APOBEC3-induced factors on prognosis varies among cancer types. APOBEC3s can affect tumorigenesis through mutagenic and non-mutagenic pathways, which may have opposing effects on disease course. The relative strength of these effects may determine the prognostic significance of APOBEC3s, explaining the varied clinical impact of APOBEC3s by cancer type (Table 5). Examples for specific cancer types are shown.
APOBEC3s have similarly deleterious effects in traditionally treated lung cancers. Outside of the immunotherapy context, multiple studies have found that APOBEC3B expression or induced mutation burden is associated with aggressive disease or poor prognosis (Table 5). In lung cancer, additional factors such as APOBEC3-induced mutations that promote therapy resistance are thus likely to outweigh APOBEC3-mediated immune activation to drive poor clinical outcomes [141] (Fig. 4).
Association with favorable immunotherapy and platinum-based treatment outcomes
In lung cancer, APOBEC3-mediated immune activation has been associated with improved responsiveness to immune checkpoint blockade therapies (Table 5). Similar results have been observed for immunotherapy response in breast, bladder, and lung cancers, which have all shown immune activation with APOBEC3s (Tables 4 and 5). In these cancers, APOBEC3-mediated immune activation likely synergizes with a high APOBEC3-induced neoantigen load to drive immunotherapy responsiveness (Fig. 4) [164, 187]. APOBEC3s are also associated with improved response to immunotherapy in gastric cancer, but this cancer type has paradoxically shown immunosuppression with APOBEC3s [37].
Although current data are somewhat unclear, APOBEC3s may also facilitate improved bladder cancer survival even with traditional chemotherapy (Table 5). Bladder cancer is often treated with the DNA alkylating agent cisplatin, which may have enhanced activity in the presence of APOBEC3s. Mechanistically, APOBEC3s may mediate cisplatin response by deaminating drug-induced extrahelical cytosines, and APOBEC3B may induce further genotoxic effects during chemotherapy-induced mismatch repair [188]. Additionally, APOBEC3s may generate a high background mutation load that sensitizes cells to additional cisplatin-induced DNA damage. However, the clinical benefit of APOBEC3s in bladder cancer could be limited to later-stage disease since higher APOBEC3B expression may contribute to the progression from non-muscle-invasive to muscle-invasive disease [1].
APOBEC3s have been similarly associated with improved clinical outcomes in ovarian cancers, which are treated like bladder cancer using the platinum-based therapies cisplatin and carboplatin (Table 5). For example, a high APOBEC3-induced mutation load was associated with improved progression-free and overall survival in clear cell ovarian carcinoma patients [186]. High APOBEC3 expression correlated with improved survival in high-grade serous ovarian tumor subtypes [41, 42, 167]. Like in bladder cancer, these APOBEC3-associated survival benefits in ovarian cancer likely arise from immune activation and increased responsiveness to platinum-based therapies (Fig. 4).
APOBEC3s as biomarkers
APOBEC3 expression and APOBEC3-induced mutation burden have been associated with clinical outcomes in multiple cancers and treatment contexts, which rationalizes their use as prognostic biomarkers (Table 5). Several studies of cisplatin/carboplatin-treated cancers have reported associations between APOBEC3s and favorable outcomes, so APOBEC3s merit further evaluation as biomarkers for this common chemotherapy [41, 46, 61, 167, 170, 186]. APOBEC3s could also be used as biomarkers for immunotherapy given existing data in lung, bladder, and breast cancers [37, 160, 166, 174, 180, 181]. In addition to these clinical associations, multiple pre-clinical studies have bolstered the case for APOBEC3s as potential platinum-based therapy and immunotherapy biomarkers (Table 6).
Table 6.
Biomarker and therapeutic potential of APOBEC3s
| Treatment or tumor context | Emerging evidence | APOBEC3 metric* | References | |
|---|---|---|---|---|
|
Biomarker: Predict strong responsiveness Therapeutic strategy: Activation of APOBEC3s to sensitize tumor cells to treatment |
Immunotherapy | Better response to immune checkpoint blockade therapy in bladder, HNSCC, breast, and lung cancer | Mutation burden and expression | [37, 160, 166, 174, 180, 181] |
| Increased PD-L1 expression | Expression (3B) | [36] | ||
| Higher PD-L1 and PD-L2 levels in glioma | Expression | [51] | ||
| Slightly higher sPD-L levels in breast cancer | Expression (3A) | [64] | ||
| A3A, A3D, and A3H correlated with PD-L1 on tumor-infiltrating immune cells, and A3F associated with more PD-L1 on tumor cells | Expression (3A, 3D, 3H, and 3F) | [171] | ||
| APOBEC3 overexpression and APOBEC3-induced kataegis positively correlated with PD-L1 expression | In vitro overexpression | [189] | ||
| Correlation with PD-L1 expression due to induction of PD-L1 via JNK/c-JUN signaling | In vitro overexpression, mutation burden and expression (3A) | [190] | ||
| Increased immune checkpoint blockade responsiveness in a melanoma model | In vivo overexpression (3B) | [187] | ||
| Improved response to anti-CTLA-4 therapy in a HER2-driven breast cancer model | In vivo overexpression | [164] | ||
| Platinum-based chemotherapy | Better survival in bladder cancer, which is often treated with cisplatin** | Mutation burden and expression | [46, 61, 170] | |
| Improved outcomes in ovarian cancer, which is typically treated with cisplatin or carboplatin** | Mutation burden and expression | [41, 167, 186] | ||
| Increased response to cisplatin in breast cancer cells | In vitro overexpression | [188] | ||
| DDR inhibitors | Increased responsiveness to ATR and Chk1/2 inhibitors in leukemia cells | In vitro overexpression (3A) | [52] | |
| Higher sensitivity to ATR and Chk1/2 inhibition | In vitro overexpression | [191] | ||
| Greater sensitivity to CHEK1/2 PARP, and WEE1 inhibition in p53-defecient T cells | In vitro overexpression | [99] | ||
| Enhanced sensitivity to PARP inhibitors in pancreatic cancer cells | In vitro overexpression (3A) | [157] | ||
| Tumors with DNA repair deficits | Induction of apoptosis, likely due to high levels of DNA damage | In vitro overexpression (3A) | [43] | |
|
Biomarker: Predict resistance Therapeutic strategy: Inhibition of APOBEC3s to promote therapeutic response or delay resistance |
Rapidly evolving tumors | APOBEC3s identified as drivers of tumor heterogeneity and subclonal evolution | N/A | [82] |
| APOBEC3-induced subclonal driver mutations in pan-cancer analysis | N/A | [148] | ||
| EGFR inhibitors | APOBEC3s may induce the T790M mutation that confers resistance to EGFR inhibitors in lung cancer | N/A | [82, 141] | |
| Raf inhibitors | Poor response to Raf inhibitors in glioma | Expression | [51] | |
| APOBEC3-induced mutations may decrease response to Raf inhibitors in multiple myeloma | N/A | [158] | ||
| APOBEC3-induced MKE2 L46F promotes resistance to BRAF inhibitors in melanoma | N/A | [82, 142] | ||
| Akt inhibitors | Increased Akt-mediated anoikis inhibition | In vitro overexpression | [49] | |
| Endocrine therapies | Accelerated resistance to tamoxifen in breast cancer cells through a catalytic mechanism | In vitro overexpression | [192] | |
| Oncolytic virotherapy | Escape from vesicular stomatitis virus therapy in melanoma models | In vitro and in vivo overexpression | [193] | |
| Decreased efficacy of oncolytic virus therapy | In vitro and in vivo overexpression | [194] | ||
| Recurrent APOBEC3-induced mutations un resistant cells | In vitro and in vivo overexpression | [187] |
*Where applicable, “mutation burden” refers to APOBEC3-induced mutation burden
**Some studies have reported mixed results (see Table 5)
Moreover, APOBEC3s could be particularly fruitful biomarkers for targeted therapies (Table 6). High APOBEC3B expression has been associated with poor response to Raf inhibitors in glioma, and certain APOBEC3-induced mutations may predict resistance to Raf inhibitors and EGFR inhibitors in multiple myeloma and lung cancer, respectively [82, 158]. In ER+ breast cancer, high APOBEC3B expression can predict resistance to the endocrine therapy tamoxifen [192]. Based on pre-clinical studies, APOBEC3s may also predict a favorable response to targeted replication checkpoint and DDR inhibitors, likely due to synthetic vulnerabilities that induce cytotoxic DNA damage [52, 99, 157, 191].
Overall, there is compelling evidence that testing patient tumors for APOBEC3 expression or induced mutation burden could guide therapy decisions. However, there is a significant gap from laboratory to clinic, and it will be essential to establish meaningful parameters that define “APOBEC-positivity.” To this end, future studies will need to evaluate APOEBC3 expression or mutational signature scores that can eventually be used in clinical trials. The scoring and profiling systems for PD-L1/PD1 and overall tumor mutation burden can be used for guidance [195–199].
Inhibition of APOBEC3s for cancer therapeutics
Since APOBEC3s can drive tumorigenesis through mutagenic and not mutagenic pathways, it could be beneficial to inhibit APOBEC3s in specific but likely numerous oncogenic contexts (Table 6). For example, APOBEC3 inhibitors could potentially prevent non-muscle invasive bladder cancer from progressing to muscle-invasive disease, which has a higher APOBEC3-induced mutation burden [1, 200]. APOBEC3 inhibitors could also slow STING-dependent metastasis, especially in combination with emerging STING inhibitors [157, 201]. Inhibiting APOBEC3G could also support anoikis since APOBEC3s have been shown to disrupt this form of cell death [49]. Additionally, APOBEC3 inhibitors could be used especially in cancers where APOBEC3s predict poor prognosis, to limit tumor evolution, subclone heterogeneity, and chemotherapy resistance [82, 148].
APOBEC3 inhibitors are not currently available for clinical use, but recent advancements in resolving the molecular structures of APOBEC3 enzymes have laid important groundwork for the eventual development of this drug class [202]. Multiple compounds with catechol moieties have been shown to inhibit APOBEC3G, with chemical modifications supporting more limited targeting of APOBEC3A [203]. A recently identified small molecule inhibitor that can target catalytic pockets of AID, APOBEC3A and APOBEC3B has also been reported and may guide the design of inhibitors specific to each APOBEC enzyme [205]. In addition to small molecule inhibitors, other potential strategies to reduce APOBEC3 activity include gene-silencing therapies, ssDNA-containing 2′-deoxyzebularine analogues, and alternative splicing modulators [204]. The latter approach is possible because some APOBEC3 isoforms are non-mutagenic, and SF3B1 inhibition has been shown to shift expression to a non-mutagenic APOBEC3B isoform by inducing exon 5 skipping [1]. Additionally, alleviation of replication stress via nucleoside supplementation and chk1 inhibition has been shown to reduce APOBEC3B expression in vitro [90].
Activation of APOBEC3s for cancer therapeutics
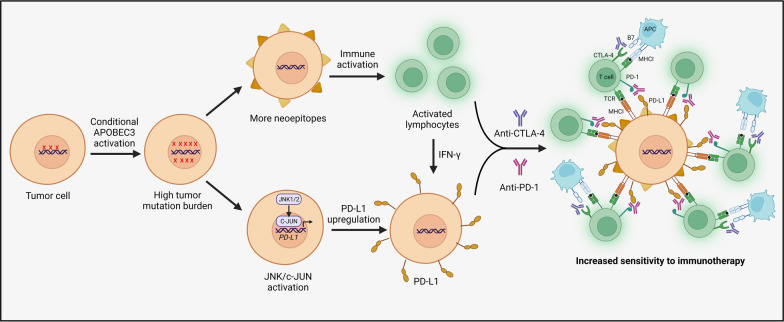
While inhibiting APOBEC3s may be beneficial in many cancers, the seemingly counterintuitive strategy of increasing APOBEC3 activity in certain contexts might offer a unique therapeutic opportunity. For example, targeted overexpression of APOEBC3s may be advantageous with immunotherapy agents. Supporting this approach, APOBEC3B induction has been shown to increase responsiveness to immune checkpoint blockade therapy in mouse models of melanoma and breast cancer [164, 187]. This APOBEC3-induced sensitization may occur through multiple synergistic pathways (Fig. 5). First, APOBEC3s can generate a high overall tumor mutation burden, leading to neoepitope formation and immune activation in some tumor types [187]. Second, APOBEC3-mediated increases in PD-L1 expression can also enhance immunotherapy response [64, 171, 189]. Mechanistically, APOBEC3s may promote PD-L1 expression through DNA damage that activates JNK/c-JUN signaling [190]. Synergies between APOBEC3s and IFN-γ may further increase PD-L1 expression on cancer cells and additively enhance sensitization to immunotherapy [206, 207].
Fig. 5.
Activation of APOBEC3s to enhance response to immunotherapy. Increasing APOBEC3 activity could increase immunotherapy response through neoepitope formation, increased PD-L1 expression, and immune activation as has been demonstrated by studies using mouse models
Since APOBEC3s induce a high level of DNA damage, their targeted overexpression in cancer cells may also be cytotoxic [208]. Activation of APOBEC3A may specifically cause apoptosis in vitro, as it has the highest deamination activity among APOBEC3s [43, 108]. Moreover, targeted overexpression of APOBEC3s may be especially cytotoxic to tumors with impaired uracil glycosylase function or loss of the abasic site sensor HCES—mutations that would create synthetic vulnerability [209, 210].
APOBEC3-activating therapies could also effectuate the use of genotoxic drugs. For example, APOBEC3 inducers could prime tumors to respond to platinum-based chemotherapies and DDR inhibitors. As a proof of concept, an in vitro breast cancer study found that inducing APOBEC3s in cell lines with low baseline expression significantly increased responsiveness to cisplatin [188]. Overexpression of APOBEC3s was also shown to increase responsiveness to targeted ATR and Chk1/2 inhibitors in acute myeloid leukemia and osteosarcoma cell lines [52, 191]. Similarly, APOBEC3B overexpression sensitized p53-deficient cells to CHEK1/2, WEE1, and PARP inhibition [99]. Increased sensitivity to PARP inhibitors was also observed with APOBEC3A upregulation in pancreatic cancer cells [157].
Despite having therapeutic potential in some contexts, APOBEC3 induction may not facilitate immunotherapy response in all cancers, especially in tumors where higher APOBEC3 expression has been associated with immunosuppression (Table 4). APOBEC3-enhancing drugs must also be explored with caution given the risks of new driver mutations, increased tumor heterogeneity, and chemotherapy resistance. Nonetheless, activation and inhibition of APOBEC3 activity are both promising approaches that could create new treatment options for tomorrow’s clinical landscape.
Conclusions
APOBEC3s are major mutagenic drivers in cancer, and APOBEC3A and APOBEC3B are likely the most significant mutators among the APOBEC3 superfamily. APOBEC3A and APOBEC3B are overexpressed in many cancers, and their expression correlates with a higher APOBEC3-induced mutation burden. However, the detection of residual deamination activity after knocking out both family members suggests that other APOBEC3s are also active mutators. An improved understanding of how each APOBEC3 contributes to cancer will further elucidate tumor etiology and may become therapeutically relevant for targeting APOBEC3s.
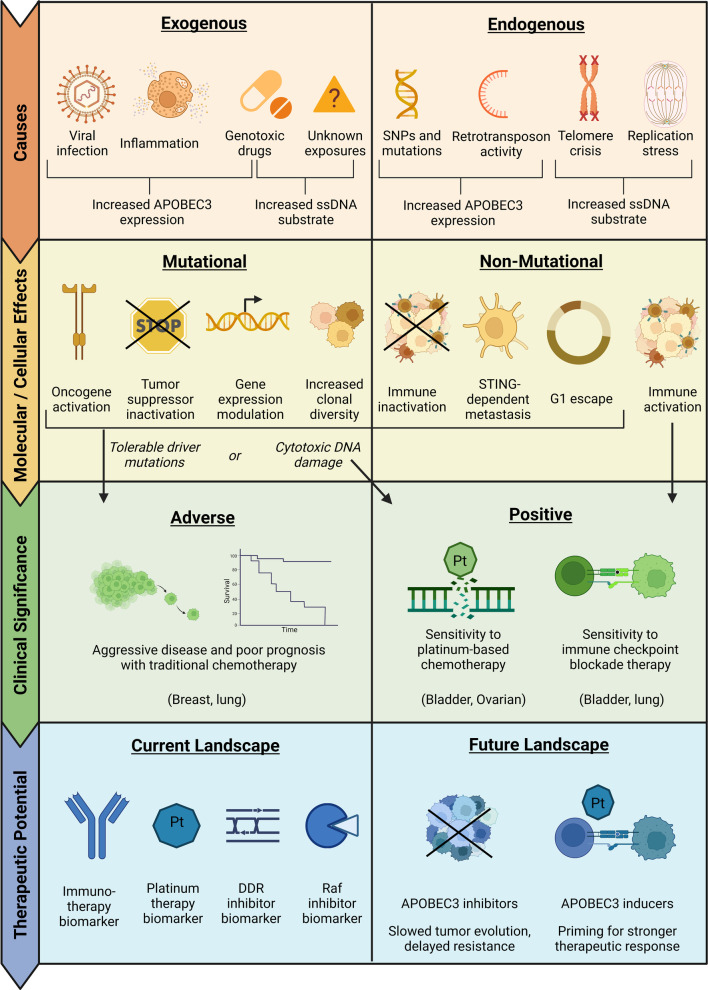
A more comprehensive understanding of the factors that induce APOBEC3-mediated mutagenesis could also lead to improved cancer prevention strategies. Currently known triggers of APOBEC3 expression include viral infection, inflammation, and certain genotoxic drugs, although increased expression alone may not cause high levels of mutagenesis (Fig. 6). In a two-factor model, ssDNA—which can arise from genotoxic drug exposure, genomic instability, etc.—creates prime mutational substrates for APOBEC3s and facilitates mutagenesis.
Fig. 6.
Causes, consequences, and clinical aspects of APOBEC3-mediated mutagenesis in cancer. A variety of exogenous and endogenous factors can induce APOBEC3s, which can then impact cancer growth through mutagenic and non-mutagenic pathways. These APOBEC3-mediated effects may shape clinical outcomes and be relevant in both the current and future therapeutic landscapes.
Although mutagenesis is central to the role of APOBEC3s in cancer, APOBEC3s can also contribute to tumorigenesis through non-mutagenic pathways such as cell cycle modulation, epigenetic regulation, STING-dependent metastasis, and cell death inhibition (Fig. 6). Additionally, APOBEC3s can modulate the tumor immune microenvironment, with the effect varying by cancer type. APOBEC3s have been associated with immune-activation in breast, lung, and bladder cancer, while APOBEC3-mediated immunosuppression has been observed in liver cancer, gastric cancer, and adrenocortical carcinoma.
The immune-modulating effects of APOBEC3s likely impact disease course, but APOBEC3-mediated immune-activation does not always translate to improved clinical outcomes since other factors such as driver mutations and cell death inhibition can have opposing effects. APOBEC3-mediated immune activation, increased neoepitope load, and higher PD-L1 expression may synergize to enhance response to immunotherapies (Fig. 6). APOBEC3s may also facilitate responsiveness to platinum-based therapies.
Number of clinical associations along with pre-clinical studies suggest that APOBEC3s could be used as biomarkers for currently available immunotherapies and chemotherapies, while novel startegies for APOBEC3 activation could prime certain tumors for increased therapeutic response (Fig. 6). In contrast, inhibiting APOBEC3s could slow tumor evolution, decrease subclone heterogeneity, and prevent therapy resistance in some cancers and in context to specific treatment types. APOBEC3s are dysregulated in a wide variety and high proportion of human tumors, so research in these areas holds great potential and could lead to new treatment options for many caner types.
Acknowledgements
We thank Alexandra Dobbins, research intern in the Banday Lab, for assisting with preliminary literature searches in PubMed related to APOBECs and cross-checking correct citations of the literature and summaries of tables. All the figures were created with BioRender.com.
Abbreviations
- APOBECs
Apolipoprotein B mRNA-editing enzyme, catalytic polypeptides
- BKPyV
BK polyomavirus
- TpC
Thiamine preceding cytosine
- DDR
DNA damage response
- ER
Estrogen receptor
- HNSCC
Head and neck squamous cell carcinoma
- HR
Homologous recombination
- HPV
Human papilloma virus
- IFN-γ
Interferon gamma
- MDSCs
Myeloid-derived suppressor cells
- NHEJ
Non-homologous end joining
- TAMs
Tumor-associated macrophages
- TNF-α
Tumor necrosis factor alpha
- TLS
Translesion synthesis
- ssDNA
Single-stranded DNA
- SNP
Single-nucleotide polymorphism
Author contributions
KB and RB conceptualized the design and wrote the manuscript. KB prepared the figures. Both authors read and approved the final manuscript.
Funding
Open Access funding provided by the National Institutes of Health (NIH). The authors are funded by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rouf Banday A, Onabajo OO, Lin SHY, Obajemu A, Vargas JM, Delviks-Frankenberry KA, et al. Targeting natural splicing plasticity of APOBEC3B restricts its expression and mutagenic activity. Commun Biol. 2021;4(1):386. doi: 10.1038/s42003-021-01844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebrahimi D, Richards CM, Carpenter MA, Wang J, Ikeda T, Becker JT, et al. Genetic and mechanistic basis for APOBEC3H alternative splicing, retrovirus restriction, and counteraction by HIV-1 protease. Nat Commun. 2018;9(1):4137. doi: 10.1038/s41467-018-06594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harari A, Ooms M, Mulder LCF, Simon V. Polymorphisms and splice variants influence the antiretroviral activity of human APOBEC3H. J Virol. 2009;83(1):295–303. doi: 10.1128/JVI.01665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lassen KG, Wissing S, Lobritz MA, Santiago M, Greene WC. Identification of two APOBEC3F splice variants displaying HIV-1 antiviral activity and contrasting sensitivity to Vif*. J Biol Chem. 2010;285(38):29326–29335. doi: 10.1074/jbc.M110.154054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salter JD, Bennett RP, Smith HC. The APOBEC protein family: united by structure, divergent in function. Trends Biochem Sci. 2016;41(7):578–594. doi: 10.1016/j.tibs.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhuri J, Evans T, Kumar R, DiMenna L. Biological function of activation-induced cytidine deaminase (AID) Biomed J. 2014;37(5):269. doi: 10.4103/2319-4170.128734. [DOI] [PubMed] [Google Scholar]
- 7.Chen SH, Habib G, Yang CY, Gu ZW, Lee BR, Weng SA, et al. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987;238(4825):363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- 8.Powell LM, Wallis SC, Pease RJ, Edwards YH, Knott TJ, Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- 9.Hirano K, Min J, Funahashi T, Baunoch DA, Davidson NO. Characterization of the human apobec-1 gene: expression in gastrointestinal tissues determined by alternative splicing with production of a novel truncated peptide. J Lipid Res. 1997;38(5):847–859. doi: 10.1016/S0022-2275(20)37210-2. [DOI] [PubMed] [Google Scholar]
- 10.Harris RS, Dudley JP. APOBECs and virus restriction. Virology. 2015;479–480:131–145. doi: 10.1016/j.virol.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvas T, Schiffer CA. APOBEC3s: DNA-editing human cytidine deaminases. Protein Sci. 2019 doi: 10.1002/pro.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Q, König R, Pillai S, Chiles K, Kearney M, Palmer S, et al. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat Struct Mol Biol. 2004;11(5):435–442. doi: 10.1038/nsmb758. [DOI] [PubMed] [Google Scholar]
- 13.Chan K, Sterling JF, Roberts SA, Bhagwat AS, Resnick MA, Gordenin DA. Base damage within single-strand DNA underlies in vivo hypermutability induced by a ubiquitous environmental agent. PLoS Genet. 2012;8(12):e1003149. doi: 10.1371/journal.pgen.1003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoopes JI, Cortez LM, Mertz TM, Malc EP, Mieczkowski PA, Roberts SA. APOBEC3A and APOBEC3B preferentially deaminate the lagging strand template during DNA replication. Cell Rep. 2016;14(6):1273–1282. doi: 10.1016/j.celrep.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lada AG, Kliver SF, Dhar A, Polev DE, Masharsky AE, Rogozin IB, et al. Disruption of transcriptional coactivator Sub1 leads to genome-wide re-distribution of clustered mutations induced by APOBEC in active yeast genes. PLoS Genet. 2015;11(5):e1005217. doi: 10.1371/journal.pgen.1005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seplyarskiy VB, Soldatov RA, Popadin KY, Antonarakis SE, Bazykin GA, Nikolaev SI. APOBEC-induced mutations in human cancers are strongly enriched on the lagging DNA strand during replication. Genome Res. 2016;26(2):174–182. doi: 10.1101/gr.197046.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buisson R, Langenbucher A, Bowen D, Kwan EE, Benes CH, Zou L, et al. Passenger hotspot mutations in cancer driven by APOBEC3A and mesoscale genomic features. Science. 2019;364:6447. doi: 10.1126/science.aaw2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCann JL, Cristini A, Law EK, Lee SY, Tellier M, Carpenter MA, et al. R-loop homeostasis and cancer mutagenesis promoted by the DNA cytosine deaminase APOBEC3B. bioRxiv [Internet]. 2021;2021.08.30.458235. Available from: http://biorxiv.org/content/early/2021/08/30/2021.08.30.458235.abstract.
- 19.Holtz CM, Sadler HA, Mansky LM. APOBEC3G cytosine deamination hotspots are defined by both sequence context and single-stranded DNA secondary structure. Nucleic Acids Res. 2013;41(12):6139–6148. doi: 10.1093/nar/gkt246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris RS. Molecular mechanism and clinical impact of APOBEC3B-catalyzed mutagenesis in breast cancer. Breast Cancer Res. 2015;17(1):8. doi: 10.1186/s13058-014-0498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Refsland EW, Harris RS. The APOBEC3 family of retroelement restriction factors. Berlin: Springer; 2013. pp. 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burns MB, Lackey L, Carpenter MA, Rathore A, Land AM, Leonard B, et al. APOBEC3B is an enzymatic source of mutation in breast cancer. Nature. 2013;494(7437):366–370. doi: 10.1038/nature11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan K, Roberts SA, Klimczak LJ, Sterling JF, Saini N, Malc EP, et al. An APOBEC3A hypermutation signature is distinguishable from the signature of background mutagenesis by APOBEC3B in human cancers. Nat Genet. 2015;47(9):1067–1072. doi: 10.1038/ng.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.COSMIC. 2022.
- 25.Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578(7793):94–101. doi: 10.1038/s41586-020-1943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin A, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts SA, Lawrence MS, Klimczak LJ, Grimm SA, Fargo D, Stojanov P, et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet. 2013;45(9):970–976. doi: 10.1038/ng.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou J, Wang C, Ma X, Wang E, Peng G. APOBEC3B, a molecular driver of mutagenesis in human cancers. Cell Biosci. 2017;7(1):29. doi: 10.1186/s13578-017-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor BJ, Nik-Zainal S, Wu YL, Stebbings LA, Raine K, Campbell PJ, et al. DNA deaminases induce break-associated mutation showers with implication of APOBEC3B and 3A in breast cancer kataegis. Elife. 2013;16:2. doi: 10.7554/eLife.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Newhall KP, Khani F, Barlow L, Nguyen D, Gu L, et al. The cytidine deaminase APOBEC3G contributes to cancer mutagenesis and clonal evolution in bladder cancer. Cancer Res. 2022;26:2651. doi: 10.1158/0008-5472.CAN-22-2912. [DOI] [PubMed] [Google Scholar]
- 31.Aaltonen LA, Abascal F, Abeshouse A, Aburatani H, Adams DJ, Agrawal N, et al. Pan-cancer analysis of whole genomes. Nature. 2020;578(7793):82–93. doi: 10.1038/s41586-020-1969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergstrom EN, Luebeck J, Petljak M, Khandekar A, Barnes M, Zhang T, et al. Mapping clustered mutations in cancer reveals APOBEC3 mutagenesis of ecDNA. Nature. 2022;602(7897):510–517. doi: 10.1038/s41586-022-04398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langenbucher A, Bowen D, Sakhtemani R, Bournique E, Wise JF, Zou L, et al. An extended APOBEC3A mutation signature in cancer. Nat Commun. 2021;12(1):1602. doi: 10.1038/s41467-021-21891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burns MB, Temiz NA, Harris RS. Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat Genet. 2013;45(9):977–983. doi: 10.1038/ng.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H, Kim O, Lee MA, Lee JY, Hong SH, Ha US, et al. Prognostic impact of APOBEC3B expression in metastatic urothelial carcinoma and its association with tumor-infiltrating cytotoxic T cells. Curr Oncol. 2021;28(3):1652–1662. doi: 10.3390/curroncol28030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Jia M, He Z, Liu XS. APOBEC3B and APOBEC mutational signature as potential predictive markers for immunotherapy response in non-small cell lung cancer. Oncogene. 2018;37(29):3924–3936. doi: 10.1038/s41388-018-0245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia S, Gu Y, Zhang H, Fei Y, Cao Y, Fang H, et al. Immune inactivation by APOBEC3B enrichment predicts response to chemotherapy and survival in gastric cancer. Oncoimmunology. 2021 doi: 10.1080/2162402X.2021.1975386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Wei W, Jin HC, Ying RC, Zhu AK, Zhang FJ. The roles of APOBEC3B in gastric cancer. Int J Clin Exp Pathol. 2015;8(5):5089–5096. [PMC free article] [PubMed] [Google Scholar]
- 39.Kosumi K, Baba Y, Ishimoto T, Harada K, Nakamura K, Ohuchi M, et al. APOBEC3B is an enzymatic source of molecular alterations in esophageal squamous cell carcinoma. Med Oncol. 2016;33(3):26. doi: 10.1007/s12032-016-0739-7. [DOI] [PubMed] [Google Scholar]
- 40.Feng C, Zheng Q, Yang Y, Xu M, Lian Y, Huang J, et al. APOBEC3B high expression in gastroenteropancreatic neuroendocrine neoplasms and association with lymph metastasis. Appl Immunohistochem Mol Morphol. 2019;27(8):599–605. doi: 10.1097/PAI.0000000000000695. [DOI] [PubMed] [Google Scholar]
- 41.Rüder U, Denkert C, Alisa Kunze C, Jank P. APOBEC3B protein expression and mRNA analyses in patients with high-grade serous ovarian carcinoma. Histol Histopathol. 2019;34:405–417. doi: 10.14670/HH-18-050. [DOI] [PubMed] [Google Scholar]
- 42.Du Y, Tao X, Wu J, Yu H, Yu Y, Zhao H. APOBEC3B up-regulation independently predicts ovarian cancer prognosis: a cohort study. Cancer Cell Int. 2018;18(1):78. doi: 10.1186/s12935-018-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu W, Ji H, Zhao J, Song J, Zheng S, Chen L, et al. Transcriptional repression and apoptosis influence the effect of <scp>APOBEC3A</scp> / <scp>3B</scp> functional polymorphisms on biliary tract cancer risk. Int J Cancer. 2022;150(11):1825–1837. doi: 10.1002/ijc.33930. [DOI] [PubMed] [Google Scholar]
- 44.Gara SK, Tyagi MV, Patel DT, Gaskins K, Lack J, Liu Y, et al. GATA3 and APOBEC3B are prognostic markers in adrenocortical carcinoma and APOBEC3B is directly transcriptionally regulated by GATA3. Oncotarget. 2020;11(36):3354–3370. doi: 10.18632/oncotarget.27703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagener R, Alexandrov LB, Montesinos-Rongen M, Schlesner M, Haake A, Drexler HG, et al. Analysis of mutational signatures in exomes from B-cell lymphoma cell lines suggest APOBEC3 family members to be involved in the pathogenesis of primary effusion lymphoma. Leukemia. 2015;29(7):1612–1615. doi: 10.1038/leu.2015.22. [DOI] [PubMed] [Google Scholar]
- 46.Glaser AP, Fantini D, Wang Y, Yu Y, Rimar KJ, Podojil JR, et al. APOBEC-mediated mutagenesis in urothelial carcinoma is associated with improved survival, mutations in DNA damage response genes, and immune response. Oncotarget. 2018;9(4):4537–4548. doi: 10.18632/oncotarget.23344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cortez LM, Brown AL, Dennis MA, Collins CD, Brown AJ, Mitchell D, et al. APOBEC3A is a prominent cytidine deaminase in breast cancer. PLoS Genet. 2019;15(12):e1008545. doi: 10.1371/journal.pgen.1008545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asaoka M, Patnaik SK, Ishikawa T, Takabe K. Different members of the APOBEC3 family of DNA mutators have opposing associations with the landscape of breast cancer. Am J Cancer Res. 2021;11:5111. [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Pan TH, Xu S, Jia LT, Zhu LL, Mao JS, et al. The virus-induced protein APOBEC3G inhibits anoikis by activation of Akt kinase in pancreatic cancer cells. Sci Rep. 2015;5(1):12230. doi: 10.1038/srep12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding Q, Chang CJ, Xie X, Xia W, Yang JY, Wang SC, et al. APOBEC3G promotes liver metastasis in an orthotopic mouse model of colorectal cancer and predicts human hepatic metastasis. J Clin Investig. 2011;121(11):4526–4536. doi: 10.1172/JCI45008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo C, Wang S, Liao W, Zhang S, Xu N, Xie W, et al. Upregulation of the APOBEC3 family is associated with a poor prognosis and influences treatment response to Raf inhibitors in low grade glioma. Int J Mol Sci. 2021;22(19):10390. doi: 10.3390/ijms221910390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green AM, Budagyan K, Hayer KE, Reed MA, Savani MR, Wertheim GB, et al. Cytosine deaminase APOBEC3A sensitizes leukemia cells to inhibition of the DNA replication checkpoint. Cancer Res. 2017;77(17):4579–4588. doi: 10.1158/0008-5472.CAN-16-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waters CE, Saldivar JC, Amin ZA, Schrock MS, Huebner K. FHIT loss-induced DNA damage creates optimal APOBEC substrates: insights into APOBEC-mediated mutagenesis. Oncotarget. 2015;6(5):3409–3419. doi: 10.18632/oncotarget.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasaki H, Suzuki A, Tatematsu T, Shitara M, Hikosaka Y, Okuda K, et al. APOBEC3B gene overexpression in non-small-cell lung cancer. Biomed Rep. 2014;2(3):392–395. doi: 10.3892/br.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng C, Zhang Y, Huang J, Zheng Q, Yang Y, Xu B. The prognostic significance of APOBEC3B and PD-L1/PD-1 in nasopharyngeal carcinoma. Appl Immunohistochem Mol Morphol. 2021;29(3):239–244. doi: 10.1097/PAI.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 56.Leonard B, Hart SN, Burns MB, Carpenter MA, Temiz NA, Rathore A, et al. APOBEC3B upregulation and genomic mutation patterns in serous ovarian carcinoma. Cancer Res. 2013;73(24):7222–7231. doi: 10.1158/0008-5472.CAN-13-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heller M, Prigge ES, Kaczorowski A, von Knebel Doeberitz M, Hohenfellner M, Duensing S. APOBEC3A expression in penile squamous cell carcinoma. Pathobiology. 2018;85(3):169–178. doi: 10.1159/000479007. [DOI] [PubMed] [Google Scholar]
- 58.Petljak M, Dananberg A, Chu K, Bergstrom EN, Striepen J, von Morgen P, et al. Mechanisms of APOBEC3 mutagenesis in human cancer cells. Nature. 2022;607(7920):799–807. doi: 10.1038/s41586-022-04972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petljak M, Maciejowski J. Molecular origins of APOBEC-associated mutations in cancer. DNA Repair (Amst) 2020;94:102905. doi: 10.1016/j.dnarep.2020.102905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carpenter MA, Li M, Rathore A, Lackey L, Law EK, Land AM, et al. Methylcytosine and normal cytosine deamination by the foreign DNA restriction enzyme APOBEC3A. J Biol Chem. 2012;287(41):34801–34808. doi: 10.1074/jbc.M112.385161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Middlebrooks CD, Banday AR, Matsuda K, Udquim KI, Onabajo OO, Paquin A, et al. Association of germline variants in the APOBEC3 region with cancer risk and enrichment with APOBEC-signature mutations in tumors. Nat Genet. 2016;48(11):1330–1338. doi: 10.1038/ng.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nik-Zainal S, Wedge DC, Alexandrov LB, Petljak M, Butler AP, Bolli N, et al. Association of a germline copy number polymorphism of APOBEC3A and APOBEC3B with burden of putative APOBEC-dependent mutations in breast cancer. Nat Genet. 2014;46(5):487–491. doi: 10.1038/ng.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuda K, Takahashi A, Middlebrooks CD, Obara W, Nasu Y, Inoue K, et al. Genome-wide association study identified SNP on 15q24 associated with bladder cancer risk in Japanese population. Hum Mol Genet. 2015;24(4):1177–1184. doi: 10.1093/hmg/ddu512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim Y, Sun DS, Yoon J, Ko YH, Won HS, Kim JS. Clinical implications of APOBEC3A and 3B expression in patients with breast cancer. PLoS ONE. 2020;15(3):e0230261. doi: 10.1371/journal.pone.0230261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Starrett GJ, Luengas EM, McCann JL, Ebrahimi D, Temiz NA, Love RP, et al. The DNA cytosine deaminase APOBEC3H haplotype I likely contributes to breast and lung cancer mutagenesis. Nat Commun. 2016;7(1):12918. doi: 10.1038/ncomms12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stavrou S, Ross SR. APOBEC3 proteins in viral immunity. J Immunol. 2015;195(10):4565–4570. doi: 10.4049/jimmunol.1501504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bosch FX, Lorincz A, Munoz N, Meijer CJLM, Shah K. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Revathidevi S, Murugan AK, Nakaoka H, Inoue I, Munirajan AK. APOBEC: a molecular driver in cervical cancer pathogenesis. Cancer Lett. 2021;496:104–116. doi: 10.1016/j.canlet.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riva G, Albano C, Gugliesi F, Pasquero S, Pacheco SFC, Pecorari G, et al. HPV meets APOBEC: new players in head and neck cancer. Int J Mol Sci. 2021;22(3):1402. doi: 10.3390/ijms22031402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferreira DA, Tayyar Y, Idris A, McMillan NAJ. A “hit-and-run” affair—a possible link for cancer progression in virally driven cancers. Biochim Biophys Acta (BBA) Rev Cancer. 2021;1875(1):188476. doi: 10.1016/j.bbcan.2020.188476. [DOI] [PubMed] [Google Scholar]
- 71.Weinreb DB, Desman GT, Amolat-Apiado MJM, Burstein DE, Godbold JH, Johnson EM. Polyoma virus infection is a prominent risk factor for bladder carcinoma in immunocompetent individuals. Diagn Cytopathol. 2006;34(3):201–203. doi: 10.1002/dc.20429. [DOI] [PubMed] [Google Scholar]
- 72.Baker SC, Mason AS, Slip RG, Skinner KT, Macdonald A, Masood O, et al. Induction of APOBEC3-mediated genomic damage in urothelium implicates BK polyomavirus (BKPyV) as a hit-and-run driver for bladder cancer. Oncogene. 2022;41(15):2139–2151. doi: 10.1038/s41388-022-02235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Starrett GJ, Buck CB. The case for BK polyomavirus as a cause of bladder cancer. Curr Opin Virol. 2019;39:8–15. doi: 10.1016/j.coviro.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009;125(8):1747–1754. doi: 10.1002/ijc.24439. [DOI] [PubMed] [Google Scholar]
- 75.Liu S, Chaudhry MR, Berrebi AA, Papadimitriou JC, Drachenberg CB, Haririan A, et al. Polyomavirus replication and smoking are independent risk factors for bladder cancer after renal transplantation. Transplantation. 2017;101(6):1488–1494. doi: 10.1097/TP.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 76.Gupta G, Kuppachi S, Kalil RS, Buck CB, Lynch CF, Engels EA. Treatment for presumed BK polyomavirus nephropathy and risk of urinary tract cancers among kidney transplant recipients in the United States. Am J Transplant. 2018;18(1):245–252. doi: 10.1111/ajt.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rogers R, Gohh R, Noska A. Urothelial cell carcinoma after BK polyomavirus infection in kidney transplant recipients: a cohort study of veterans. Transpl Infect Dis. 2017;19(5):e12752. doi: 10.1111/tid.12752. [DOI] [PubMed] [Google Scholar]
- 78.Kenan DJ, Mieczkowski PA, Burger-Calderon R, Singh HK, Nickeleit V. The oncogenic potential of BK-polyomavirus is linked to viral integration into the human genome. J Pathol. 2015;237(3):379–389. doi: 10.1002/path.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sirohi D, Vaske C, Sanborn Z, Smith SC, Don MD, Lindsey KG, et al. Polyoma virus-associated carcinomas of the urologic tract: a clinicopathologic and molecular study. Mod Pathol. 2018;31(9):1429–1441. doi: 10.1038/s41379-018-0065-z. [DOI] [PubMed] [Google Scholar]
- 80.Kenan DJ, Mieczkowski PA, Latulippe E, Côté I, Singh HK, Nickeleit V. BK polyomavirus genomic integration and large T antigen expression: evolving paradigms in human oncogenesis. Am J Transplant. 2017;17(6):1674–1680. doi: 10.1111/ajt.14191. [DOI] [PubMed] [Google Scholar]
- 81.Petljak M, Alexandrov LB, Brammeld JS, Price S, Wedge DC, Grossmann S, et al. Characterizing mutational signatures in human cancer cell lines reveals episodic APOBEC mutagenesis. Cell. 2019;176(6):1282–1294.e20. doi: 10.1016/j.cell.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swanton C, McGranahan N, Starrett GJ, Harris RS. APOBEC enzymes: mutagenic fuel for cancer evolution and heterogeneity. Cancer Discov. 2015;5(7):704–712. doi: 10.1158/2159-8290.CD-15-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maruyama W, Shirakawa K, Matsui H, Matsumoto T, Yamazaki H, Sarca AD, et al. Classical NF-κB pathway is responsible for APOBEC3B expression in cancer cells. Biochem Biophys Res Commun. 2016;478(3):1466–1471. doi: 10.1016/j.bbrc.2016.08.148. [DOI] [PubMed] [Google Scholar]
- 84.Covino DA, Gauzzi MC, Fantuzzi L. Understanding the regulation of APOBEC3 expression: current evidence and much to learn. J Leukoc Biol. 2018;103(3):433–444. doi: 10.1002/JLB.2MR0717-310R. [DOI] [PubMed] [Google Scholar]
- 85.Li S, Bao X, Wang D, You L, Li X, Yang H, et al. APOBEC3B and IL-6 form a positive feedback loop in hepatocellular carcinoma cells. Sci China Life Sci. 2017;60(6):617–626. doi: 10.1007/s11427-016-9058-6. [DOI] [PubMed] [Google Scholar]
- 86.Liu W, Wu J, Yang F, Ma L, Ni C, Hou X, et al. Genetic polymorphisms predisposing the interleukin 6–induced APOBEC3B-UNG imbalance increase HCC risk via promoting the generation of APOBEC-signature HBV mutations. Clin Cancer Res. 2019;25(18):5525–5536. doi: 10.1158/1078-0432.CCR-18-3083. [DOI] [PubMed] [Google Scholar]
- 87.Amcheslavsky A, Zou W, Bar-Shavit Z. Toll-like receptor 9 regulates tumor necrosis factor-α expression by different mechanisms. J Biol Chem. 2004;279(52):54039–54045. doi: 10.1074/jbc.M409138200. [DOI] [PubMed] [Google Scholar]
- 88.Siriwardena SU, Perera MLW, Senevirathne V, Stewart J, Bhagwat AS. A tumor-promoting phorbol ester causes a large increase in APOBEC3A expression and a moderate increase in APOBEC3B expression in a normal human keratinocyte cell line without increasing genomic uracils. Mol Cell Biol. 2019;39(1):1–17. doi: 10.1128/MCB.00238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roper N, Gao S, Maity TK, Banday AR, Zhang X, Venugopalan A, et al. APOBEC mutagenesis and copy-number alterations are drivers of proteogenomic tumor evolution and heterogeneity in metastatic thoracic tumors. Cell Rep. 2019;26(10):2651–2666.e6. doi: 10.1016/j.celrep.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanu N, Cerone MA, Goh G, Zalmas LP, Bartkova J, Dietzen M, et al. DNA replication stress mediates APOBEC3 family mutagenesis in breast cancer. Genome Biol. 2016;17(1):185. doi: 10.1186/s13059-016-1042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Periyasamy M, Singh AK, Gemma C, Farzan R, Allsopp RC, Shaw JA, et al. Induction of APOBEC3B expression by chemotherapy drugs is mediated by DNA-PK-directed activation of NF-κB. Oncogene. 2021;40(6):1077–1090. doi: 10.1038/s41388-020-01583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.El-Bassouny DR, Omar NM, Khalaf HA, Al-Salam RAA. Role of nuclear factor-kappa B in bleomycin induced pulmonary fibrosis and the probable alleviating role of ginsenoside: histological, immunohistochemical, and biochemical study. Anat Cell Biol. 2021;54(4):448–464. doi: 10.5115/acb.21.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mojiri-Forushani H, Hemmati AA, Khodadadi A, Rashno M. Valsartan attenuates bleomycin-induced pulmonary fibrosis by inhibition of NF-κB expression and regulation of Th1/Th2 cytokines. Immunopharmacol Immunotoxicol. 2018;40(3):225–231. doi: 10.1080/08923973.2018.1431924. [DOI] [PubMed] [Google Scholar]
- 94.Maciejowski J, Chatzipli A, Dananberg A, Chu K, Toufektchan E, Klimczak LJ, et al. APOBEC3-dependent kataegis and TREX1-driven chromothripsis during telomere crisis. Nat Genet. 2020;52(9):884–890. doi: 10.1038/s41588-020-0667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nowarski R, Kotler M. APOBEC3 cytidine deaminases in double-strand DNA break repair and cancer promotion. Cancer Res. 2013;73(12):3494–3498. doi: 10.1158/0008-5472.CAN-13-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mas-Ponte D, Supek F. DNA mismatch repair promotes APOBEC3-mediated diffuse hypermutation in human cancers. Nat Genet. 2020;52(9):958–968. doi: 10.1038/s41588-020-0674-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Venkatesan S, Angelova M, Puttick C, Zhai H, Caswell DR, Lu WT, et al. Induction of APOBEC3 exacerbates DNA replication stress and chromosomal instability in early breast and lung cancer evolution. Cancer Discov. 2021;11(10):2456–2473. doi: 10.1158/2159-8290.CD-20-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Landry S, Narvaiza I, Linfesty DC, Weitzman MD. APOBEC3A can activate the DNA damage response and cause cell-cycle arrest. EMBO Rep. 2011;12(5):444–450. doi: 10.1038/embor.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nikkilä J, Kumar R, Campbell J, Brandsma I, Pemberton HN, Wallberg F, et al. Elevated APOBEC3B expression drives a kataegic-like mutation signature and replication stress-related therapeutic vulnerabilities in p53-defective cells. Br J Cancer. 2017;117(1):113–123. doi: 10.1038/bjc.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Priestley P, Baber J, Lolkema MP, Steeghs N, de Bruijn E, Shale C, et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature. 2019;575(7781):210–216. doi: 10.1038/s41586-019-1689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elliott K, Larsson E. Non-coding driver mutations in human cancer. Nat Rev Cancer. 2021;21(8):500–509. doi: 10.1038/s41568-021-00371-z. [DOI] [PubMed] [Google Scholar]
- 102.Liu J, Sieuwerts AM, Look MP, van der Vlugt-Daane M, Meijer-van Gelder ME, Foekens JA, et al. The 29.5 kb APOBEC3B deletion polymorphism is not associated with clinical outcome of breast cancer. PLoS ONE. 2016;11(8):e0161731. doi: 10.1371/journal.pone.0161731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rothman N, Garcia-Closas M, Chatterjee N, Malats N, Wu X, Figueroa JD, et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet. 2010;42(11):978–984. doi: 10.1038/ng.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kidd JM, Newman TL, Tuzun E, Kaul R, Eichler EE. Population stratification of a common APOBEC gene deletion polymorphism. PLoS Genet. 2007;3(4):e63. doi: 10.1371/journal.pgen.0030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cescon DW, Haibe-Kains B, Mak TW. APOBEC3B expression in breast cancer reflects cellular proliferation, while a deletion polymorphism is associated with immune activation. Proc Natl Acad Sci. 2015;112(9):2841–2846. doi: 10.1073/pnas.1424869112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hix MA, Wong L, Flath B, Chelico L, Cisneros GA. Single-nucleotide polymorphism of the DNA cytosine deaminase APOBEC3H haplotype I leads to enzyme destabilization and correlates with lung cancer. NAR Cancer. 2020;2(3):zcaa023. doi: 10.1093/narcan/zcaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu M, Wang Y, Wang C, Shen W, Liu J, Geng L, et al. The eQTL-missense polymorphisms of APOBEC3H are associated with lung cancer risk in a Han Chinese population. Sci Rep. 2015;5(1):14969. doi: 10.1038/srep14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan X, Zheng S, Liu W, Liu Y, Kang Z, Li Z, et al. Effect of APOBEC3A functional polymorphism on renal cell carcinoma is influenced by tumor necrosis factor-α and transcriptional repressor ETS1. Am J Cancer Res. 2021;11(9):4347–4363. [PMC free article] [PubMed] [Google Scholar]
- 109.Wen WX, Soo JSS, Kwan PY, Hong E, Khang TF, Mariapun S, et al. Germline APOBEC3B deletion is associated with breast cancer risk in an Asian multi-ethnic cohort and with immune cell presentation. Breast Cancer Res. 2016;18(1):56. doi: 10.1186/s13058-016-0717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gansmo LB, Sofiyeva N, Bjørnslett M, Romundstad P, Hveem K, Vatten L, et al. Impact of the APOBEC3A/B deletion polymorphism on risk of ovarian cancer. Sci Rep. 2021;11(1):23463. doi: 10.1038/s41598-021-02820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Komatsu A, Nagasaki K, Fujimori M, Amano J, Miki Y. Identification of novel deletion polymorphisms in breast cancer. Int J Oncol. 2008;33(2):261–270. [PubMed] [Google Scholar]
- 112.Petljak M, Maciejowski J. The APOBEC3A deaminase drives episodic mutagenesis in cancer cells. Biorxiv. 2021.
- 113.Yang W. Structure and mechanism for DNA lesion recognition. Cell Res. 2008;18(1):184–197. doi: 10.1038/cr.2007.116. [DOI] [PubMed] [Google Scholar]
- 114.Li GM. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008;18(1):85–98. doi: 10.1038/cr.2007.115. [DOI] [PubMed] [Google Scholar]
- 115.Sung P, Klein H. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat Rev Mol Cell Biol. 2006;7(10):739–750. doi: 10.1038/nrm2008. [DOI] [PubMed] [Google Scholar]
- 116.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ, et al. Somatic mutant clones colonize the human esophagus with age. Science. 2018;362(6417):911–917. doi: 10.1126/science.aau3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moore L, Leongamornlert D, Coorens THH, Sanders MA, Ellis P, Dentro SC, et al. The mutational landscape of normal human endometrial epithelium. Nature. 2020;580(7805):640–646. doi: 10.1038/s41586-020-2214-z. [DOI] [PubMed] [Google Scholar]
- 119.Lee-Six H, Olafsson S, Ellis P, Osborne RJ, Sanders MA, Moore L, et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature. 2019;574(7779):532–537. doi: 10.1038/s41586-019-1672-7. [DOI] [PubMed] [Google Scholar]
- 120.Wang Y, Robinson PS, Coorens THH, Moore L, Lee-Six H, Noorani A, et al. APOBEC mutagenesis is a common process in normal human small intestine. Nat Genet. 2023;55(2):246–254. doi: 10.1038/s41588-022-01296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306(7):737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ozlü T, Bülbül Y. Smoking and lung cancer. Tuberk Toraks. 2005;53(2):200–209. [PubMed] [Google Scholar]
- 123.Alexandrov LB, Ju YS, Haase K, van Loo P, Martincorena I, Nik-Zainal S, et al. Mutational signatures associated with tobacco smoking in human cancer. Science. 2016;354(6312):618–622. doi: 10.1126/science.aag0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fantini D, Seiler R, Meeks JJ. Molecular footprints of muscle-invasive bladder cancer in smoking and nonsmoking patients. Urol Oncol Semin Orig Investig. 2019;37(11):818–825. doi: 10.1016/j.urolonc.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 125.Koutros S, Rao N, Moore LE, Nickerson ML, Lee D, Zhu B, et al. Targeted deep sequencing of bladder tumors reveals novel associations between cancer gene mutations and mutational signatures with major risk factors. Clin Cancer Res. 2021;27(13):3725–3733. doi: 10.1158/1078-0432.CCR-20-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3(10):733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 127.Shi MJ, Meng XY, Lamy P, Banday AR, Yang J, Moreno-Vega A, et al. APOBEC-mediated mutagenesis as a likely cause of FGFR3 S249C mutation over-representation in bladder cancer. Eur Urol. 2019;76(1):9–13. doi: 10.1016/j.eururo.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 128.Tomlinson DC, Hurst CD, Knowles MA. Knockdown by shRNA identifies S249C mutant FGFR3 as a potential therapeutic target in bladder cancer. Oncogene. 2007;26(40):5889–5899. doi: 10.1038/sj.onc.1210399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liao RG, Jung J, Tchaicha J, Wilkerson MD, Sivachenko A, Beauchamp EM, et al. Inhibitor-sensitive FGFR2 and FGFR3 mutations in lung squamous cell carcinoma. Cancer Res. 2013;73(16):5195–5205. doi: 10.1158/0008-5472.CAN-12-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chandrani P, Prabhash K, Prasad R, Sethunath V, Ranjan M, Iyer P, et al. Drug-sensitiveFGFR3 mutations in lung adenocarcinoma. Ann Oncol. 2017;28(3):597–603. doi: 10.1093/annonc/mdw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shotelersuk V, Ittiwut C, Shotelersuk K, Triratanachat S, Poovorawan Y, Mutirangura A. Fibroblast growth factor receptor 3 S249C mutation in virus associated squamous cell carcinomas. Oncol Rep. 2001;8(6):1301–1304. doi: 10.3892/or.8.6.1301. [DOI] [PubMed] [Google Scholar]
- 132.Cannataro VL, Gaffney SG, Sasaki T, Issaeva N, Grewal NKS, Grandis JR, et al. APOBEC-induced mutations and their cancer effect size in head and neck squamous cell carcinoma. Oncogene. 2019;38(18):3475–3487. doi: 10.1038/s41388-018-0657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Henderson S, Chakravarthy A, Su X, Boshoff C, Fenton TR. APOBEC-mediated cytosine deamination links PIK3CA helical domain mutations to human papillomavirus-driven tumor development. Cell Rep. 2014;7(6):1833–1841. doi: 10.1016/j.celrep.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 134.Leontiadou H, Galdadas I, Athanasiou C, Cournia Z. Insights into the mechanism of the PIK3CA E545K activating mutation using MD simulations. Sci Rep. 2018;8(1):15544. doi: 10.1038/s41598-018-27044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ross RL, Askham JM, Knowles MA. PIK3CA mutation spectrum in urothelial carcinoma reflects cell context-dependent signaling and phenotypic outputs. Oncogene. 2013;32(6):768–776. doi: 10.1038/onc.2012.87. [DOI] [PubMed] [Google Scholar]
- 136.Ranjbar R, Mohammadpour S, Torshizi Esfahani A, Namazian S, Yaghob-Taleghani M, Baghaei K, et al. Prevalence and prognostic role of PIK3CA E545K mutation in Iranian colorectal cancer patients. Gastroenterol Hepatol Bed Bench. 2019;12(Suppl1):S22–S29. [PMC free article] [PubMed] [Google Scholar]
- 137.Jiang W, He T, Liu S, Zheng Y, Xiang L, Pei X, et al. The PIK3CA E542K and E545K mutations promote glycolysis and proliferation via induction of the β-catenin/SIRT3 signaling pathway in cervical cancer. J Hematol Oncol. 2018;11(1):139. doi: 10.1186/s13045-018-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thirumal Kumar D, George Priya Doss C. Role of E542 and E545 missense mutations of PIK3CA in breast cancer: a comparative computational approach. J Biomol Struct Dyn. 2017;35(12):2745–2757. doi: 10.1080/07391102.2016.1231082. [DOI] [PubMed] [Google Scholar]
- 139.Scheffler M, Bos M, Gardizi M, König K, Michels S, Fassunke J, et al. PIK3CA mutations in non-small cell lung cancer (NSCLC): genetic heterogeneity, prognostic impact and incidence of prior malignancies. Oncotarget. 2015;6(2):1315–1326. doi: 10.18632/oncotarget.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Voutsadakis IA. The landscape of PIK3CA mutations in colorectal cancer. Clin Colorectal Cancer. 2021;20(3):201–215. doi: 10.1016/j.clcc.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 141.Camidge DR, Pao W, Sequist L. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. 2014;11(8):473–481. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- 142.van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannessen CM, Goetz EM, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4(1):94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fan J, Bellon M, Ju M, Zhao L, Wei M, Fu L, et al. Clinical significance of FBXW7 loss of function in human cancers. Mol Cancer. 2022;21(1):87. doi: 10.1186/s12943-022-01548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jeeta RR, Gordon NS, Baxter L, Goel A, Noyvert B, Ott S, et al. Non-coding mutations in urothelial bladder cancer: biological and clinical relevance and potential utility as biomarkers. Bladder Cancer. 2019;5(4):263–272. doi: 10.3233/BLC-190251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu S, Ou T, Xing N, Lu J, Wan S, Wang C, et al. Whole-genome sequencing identifies ADGRG6 enhancer mutations and FRS2 duplications as angiogenesis-related drivers in bladder cancer. Nat Commun. 2019;10(1):720. doi: 10.1038/s41467-019-08576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li Z, Abraham BJ, Berezovskaya A, Farah N, Liu Y, Leon T, et al. APOBEC signature mutation generates an oncogenic enhancer that drives LMO1 expression in T-ALL. Leukemia. 2017;31(10):2057–2064. doi: 10.1038/leu.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47–54. doi: 10.1038/nature17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McGranahan N, Favero F, de Bruin EC, Birkbak NJ, Szallasi Z, Swanton C. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci Transl Med. 2015;7(283):283ra54. doi: 10.1126/scitranslmed.aaa1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang X, Wu Z, Hao Y, Yu T, Li X, Liang Y, et al. Aberrantly activated APOBEC3B is associated with mutant p53-driven refractory/relapsed diffuse large B-cell lymphoma. Front Immunol. 2022;3:13. doi: 10.3389/fimmu.2022.888250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Periyasamy M, Singh AK, Gemma C, Kranjec C, Farzan R, Leach DA, et al. p53 controls expression of the DNA deaminase APOBEC3B to limit its potential mutagenic activity in cancer cells. Nucleic Acids Res. 2017;45(19):11056–11069. doi: 10.1093/nar/gkx721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oguchi ME, Noguchi K, Fukuda M. TBC1D12 is a novel Rab11-binding protein that modulates neurite outgrowth of PC12 cells. PLoS ONE. 2017;12(4):e0174883. doi: 10.1371/journal.pone.0174883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pignot G, le Goux C, Vacher S, Schnitzler A, Radvanyi F, Allory Y, et al. PLEKHS1: a new molecular marker predicting risk of progression of non-muscle-invasive bladder cancer. Oncol Lett. 2019;18:3471–3480. doi: 10.3892/ol.2019.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu J, Zhao M, Yuan B, Gu S, Zheng M, Zou J, et al. WDR74 functions as a novel coactivator in TGF-β signaling. J Genet Genom. 2018;45(12):639–650. doi: 10.1016/j.jgg.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 154.Li Y, Chen F, Shen W, Li B, Xiang R, Qu L, et al. WDR74 induces nuclear β-catenin accumulation and activates Wnt-responsive genes to promote lung cancer growth and metastasis. Cancer Lett. 2020;471:103–115. doi: 10.1016/j.canlet.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 155.Rheinbay E, Parasuraman P, Grimsby J, Tiao G, Engreitz JM, Kim J, et al. Recurrent and functional regulatory mutations in breast cancer. Nature. 2017;547(7661):55–60. doi: 10.1038/nature22992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jakobsdottir GM, Brewer DS, Cooper C, Green C, Wedge DC. APOBEC3 mutational signatures are associated with extensive and diverse genomic instability across multiple tumour types. BMC Biol. 2022;20(1):117. doi: 10.1186/s12915-022-01316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wörmann SM, Zhang A, Thege FI, Cowan RW, Rupani DN, Wang R, et al. APOBEC3A drives deaminase domain-independent chromosomal instability to promote pancreatic cancer metastasis. Nat Cancer. 2021;2(12):1338–1356. doi: 10.1038/s43018-021-00268-8. [DOI] [PubMed] [Google Scholar]
- 158.Vo JN, Wu YM, Mishler J, Hall S, Mannan R, Wang L, et al. The genetic heterogeneity and drug resistance mechanisms of relapsed refractory multiple myeloma. Nat Commun. 2022;13(1):3750. doi: 10.1038/s41467-022-31430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ma W, Ho DW, Sze KM, Tsui Y, Chan L, Lee JM, et al. APOBEC3B promotes hepatocarcinogenesis and metastasis through novel deaminase-independent activity. Mol Carcinog. 2019;58(5):643–653. doi: 10.1002/mc.22956. [DOI] [PubMed] [Google Scholar]
- 160.Shi R, Wang X, Wu Y, Xu B, Zhao T, Trapp C, et al. APOBEC-mediated mutagenesis is a favorable predictor of prognosis and immunotherapy for bladder cancer patients: evidence from pan-cancer analysis and multiple databases. Theranostics. 2022;12(9):4181–4199. doi: 10.7150/thno.73235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Periyasamy M, Patel H, Lai CF, Nguyen VTM, Nevedomskaya E, Harrod A, et al. APOBEC3B-mediated cytidine deamination is required for estrogen receptor action in breast cancer. Cell Rep. 2015;13(1):108–121. doi: 10.1016/j.celrep.2015.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo H, Zhu L, Huang L, Sun Z, Zhang H, Nong B, et al. APOBEC alteration contributes to tumor growth and immune escape in pan-cancer. Cancers (Basel) 2022;14(12):2827. doi: 10.3390/cancers14122827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang D, Li X, Li J, Lu Y, Zhao S, Tang X, et al. APOBEC3B interaction with PRC2 modulates microenvironment to promote HCC progression. Gut. 2019;68(10):1846–1857. doi: 10.1136/gutjnl-2018-317601. [DOI] [PubMed] [Google Scholar]
- 164.DiMarco A, Qin X, McKinney BJ, Garcia NMG, van Alsten SC, Mendes EA, et al. APOBEC mutagenesis inhibits breast cancer growth through induction of T cell–mediated antitumor immune responses. Cancer Immunol Res. 2022;10(1):70–86. doi: 10.1158/2326-6066.CIR-21-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mao Y, Lv M, Zhang Y, Nie G, Cui J, Wang Y, et al. APOBEC3B expression and its prognostic potential in breast cancer. Oncol Lett. 2020;19:3205–3214. doi: 10.3892/ol.2020.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen H, Chong W, Teng C, Yao Y, Wang X, Li X. The immune response-related mutational signatures and driver genes in non-small-cell lung cancer. Cancer Sci. 2019;110(8):2348–2356. doi: 10.1111/cas.14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Leonard B, Starrett GJ, Maurer MJ, Oberg AL, van Bockstal M, van Dorpe J, et al. APOBEC3G expression correlates with T-cell infiltration and improved clinical outcomes in high-grade serous ovarian carcinoma. Clin Cancer Res. 2016;22(18):4746–4755. doi: 10.1158/1078-0432.CCR-15-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Alqassim EY, Sharma S, Khan ANMNH, Emmons TR, Cortes Gomez E, Alahmari A, et al. RNA editing enzyme APOBEC3A promotes pro-inflammatory M1 macrophage polarization. Commun Biol. 2021;4(1):102. doi: 10.1038/s42003-020-01620-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xu L, Chang Y, An H, Zhu Y, Yang Y, Xu J. High APOBEC3B expression is a predictor of recurrence in patients with low-risk clear cell renal cell carcinoma. Urol Oncol. 2015;33(8):340.e1–8. doi: 10.1016/j.urolonc.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 170.Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540–556.e25. doi: 10.1016/j.cell.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mullane SA, Werner L, Rosenberg J, Signoretti S, Callea M, Choueiri TK, et al. Correlation of apobec Mrna expression with overall survival and pd-l1 expression in urothelial carcinoma. Sci Rep. 2016;10(6):27702. doi: 10.1038/srep27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lindskrog SV, Prip F, Lamy P, Taber A, Groeneveld CS, Birkenkamp-Demtröder K, et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat Commun. 2021;12(1):2301. doi: 10.1038/s41467-021-22465-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hedegaard J, Lamy P, Nordentoft I, Algaba F, Høyer S, Ulhøi BP, et al. Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell. 2016;30(1):27–42. doi: 10.1016/j.ccell.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 174.Miao D, Margolis CA, Vokes NI, Liu D, Taylor-Weiner A, Wankowicz SM, et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat Genet. 2018;50(9):1271–1281. doi: 10.1038/s41588-018-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jafarpour S, Yazdi M, Nedaeinia R, Ghobakhloo S, Salehi R. Unfavorable prognosis and clinical consequences of APOBEC3B expression in breast and other cancers: a systematic review and meta-analysis. Tumour Biol. 2022;44(1):153–169. doi: 10.3233/TUB-211577. [DOI] [PubMed] [Google Scholar]
- 176.Tsuboi M, Yamane A, Horiguchi J, Yokobori T, Kawabata-Iwakawa R, Yoshiyama S, et al. APOBEC3B high expression status is associated with aggressive phenotype in Japanese breast cancers. Breast Cancer. 2016;23(5):780–788. doi: 10.1007/s12282-015-0641-8. [DOI] [PubMed] [Google Scholar]
- 177.Sieuwerts AM, Willis S, Burns MB, Look MP, Gelder MEMV, Schlicker A, et al. Elevated APOBEC3B correlates with poor outcomes for estrogen-receptor-positive breast cancers. Horm Cancer. 2014;5(6):405–413. doi: 10.1007/s12672-014-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tokunaga E, Yamashita N, Tanaka K, Inoue Y, Akiyoshi S, Saeki H, et al. Expression of APOBEC3B mRNA in primary breast cancer of Japanese women. PLoS ONE. 2016;11(12):e0168090. doi: 10.1371/journal.pone.0168090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fujiki Y, Yamamoto Y, Sueta A, Yamamoto-Ibusuki M, Goto-Yamaguchi L, Tomiguchi M, et al. APOBEC3B gene expression as a novel predictive factor for pathological complete response to neoadjuvant chemotherapy in breast cancer. Oncotarget. 2018;9(55):30513–30526. doi: 10.18632/oncotarget.25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sammons S, Raskina K, Danziger N, Alder L, Schrock AB, Venstrom JM, et al. APOBEC mutational signatures in hormone receptor-positive human epidermal growth factor receptor 2–negative breast cancers are associated with poor outcomes on CDK4/6 inhibitors and endocrine therapy. JCO Precis Oncol. 2022 doi: 10.1200/PO.22.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Barroso-Sousa R, Jain E, Cohen O, Kim D, Buendia-Buendia J, Winer E, et al. Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol. 2020;31(3):387–394. doi: 10.1016/j.annonc.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 182.Jiang C, Zhang Y, Li Y, Lu J, Huang Q, Xu R, et al. High CEP55 expression is associated with poor prognosis in non-small-cell lung cancer. Onco Targets Ther. 2018;11:4979–4990. doi: 10.2147/OTT.S165750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yan S, He F, Gao B, Wu H, Li M, Huang L, et al. Increased APOBEC3B predicts worse outcomes in lung cancer: a comprehensive retrospective study. J Cancer. 2016;7(6):618–625. doi: 10.7150/jca.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Qian J, Zhao S, Zou Y, Rahman SMJ, Senosain MF, Stricker T, et al. Genomic underpinnings of tumor behavior in in situ and early lung adenocarcinoma. Am J Respir Crit Care Med. 2020;201(6):697–706. doi: 10.1164/rccm.201902-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Meng F, Zhang L, Ren Y, Ma Q. The genomic alterations of lung adenocarcinoma and lung squamous cell carcinoma can explain the differences of their overall survival rates. J Cell Physiol. 2019;234(7):10918–10925. doi: 10.1002/jcp.27917. [DOI] [PubMed] [Google Scholar]
- 186.Serebrenik AA, Argyris PP, Jarvis MC, Brown WL, Bazzaro M, Vogel RI, et al. The DNA cytosine deaminase APOBEC3B is a molecular determinant of platinum responsiveness in clear cell ovarian cancer. Clin Cancer Res. 2020;26(13):3397–3407. doi: 10.1158/1078-0432.CCR-19-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Driscoll CB, Schuelke MR, Kottke T, Thompson JM, Wongthida P, Tonne JM, et al. APOBEC3B-mediated corruption of the tumor cell immunopeptidome induces heteroclitic neoepitopes for cancer immunotherapy. Nat Commun. 2020;11(1):790. doi: 10.1038/s41467-020-14568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Conner KL, Shaik AN, Marshall KA, Floyd AM, Ekinci E, Lindquist J, et al. APOBEC3 enzymes mediate efficacy of cisplatin and are epistatic with base excision repair and mismatch repair in platinum response. NAR Cancer. 2020;2(4):1–15. doi: 10.1093/narcan/zcaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Boichard A, Tsigelny IF, Kurzrock R. High expression of PD-1 ligands is associated with kataegis mutational signature and APOBEC3 alterations. Oncoimmunology. 2017;6(3):e1284719. doi: 10.1080/2162402X.2017.1284719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhao K, Zhang Q, Flanagan SA, Lang X, Jiang L, Parsels LA, et al. Cytidine deaminase APOBEC3A regulates PD-L1 expression in cancer cells in a JNK/c-JUN-dependent manner. Mol Cancer Res. 2021;19(9):1571–1582. doi: 10.1158/1541-7786.MCR-21-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Buisson R, Lawrence MS, Benes CH, Zou L. APOBEC3A and APOBEC3B activities render cancer cells susceptible to ATR inhibition. Cancer Res. 2017;77(17):4567–4578. doi: 10.1158/0008-5472.CAN-16-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Law EK, Sieuwerts AM, LaPara K, Leonard B, Starrett GJ, Molan AM, et al. The DNA cytosine deaminase APOBEC3B promotes tamoxifen resistance in ER-positive breast cancer. Sci Adv. 2016;2(10):e1601737. doi: 10.1126/sciadv.1601737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Huff AL, Wongthida P, Kottke T, Thompson JM, Driscoll CB, Schuelke M, et al. APOBEC3 mediates resistance to oncolytic viral therapy. Mol Ther Oncolytics. 2018;11:1–13. doi: 10.1016/j.omto.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Evgin L, Huff AL, Kottke T, Thompson J, Molan AM, Driscoll CB, et al. Suboptimal T-cell therapy drives a tumor cell mutator phenotype that promotes escape from first-line treatment. Cancer Immunol Res. 2019;7(5):828–840. doi: 10.1158/2326-6066.CIR-18-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Krieger T, Pearson I, Bell J, Doherty J, Robbins P. Targeted literature review on use of tumor mutational burden status and programmed cell death ligand 1 expression to predict outcomes of checkpoint inhibitor treatment. Diagn Pathol. 2020;15(1):6. doi: 10.1186/s13000-020-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Paver EC, Cooper WA, Colebatch AJ, Ferguson PM, Hill SK, Lum T, et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: a guide to immunohistochemistry implementation and interpretation. Pathology. 2021;53(2):141–156. doi: 10.1016/j.pathol.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 197.Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18(1):248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 198.Pereira MA, Ramos MFKP, Dias AR, Ribeiro R, Cardili L, Zilberstein B, et al. Scoring systems for PD-L1 expression and their prognostic impact in patients with resectable gastric cancer. Virchows Arch. 2021;478(6):1039–1048. doi: 10.1007/s00428-020-02956-9. [DOI] [PubMed] [Google Scholar]
- 199.Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, Postel-Vinay S, et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: The Gustave Roussy Immune Score (GRIm-Score) Eur J Cancer. 2017;84:212–218. doi: 10.1016/j.ejca.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 200.Wang F, Dong X, Yang F, Xing N. Comparative analysis of differentially mutated genes in non-muscle and muscle-invasive bladder cancer in the Chinese population by whole exome sequencing. Front Genet. 2022;13:831146. doi: 10.3389/fgene.2022.831146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wu J, Zhao L, Hu H, Li W, Li Y. Agonists and inhibitors of the STING pathway: potential agents for immunotherapy. Med Res Rev. 2020;40(3):1117–1141. doi: 10.1002/med.21649. [DOI] [PubMed] [Google Scholar]
- 202.Shi K, Carpenter MA, Banerjee S, Shaban NM, Kurahashi K, Salamango DJ, et al. Structural basis for targeted DNA cytosine deamination and mutagenesis by APOBEC3A and APOBEC3B. Nat Struct Mol Biol. 2017;24(2):131–139. doi: 10.1038/nsmb.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li M, Shandilya SMD, Carpenter MA, Rathore A, Brown WL, Perkins AL, et al. First-in-class small molecule inhibitors of the single-strand DNA cytosine deaminase APOBEC3G. ACS Chem Biol. 2012;7(3):506–517. doi: 10.1021/cb200440y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kvach M, Barzak FM, Harjes S, Schares HAM, Jameson GB, Ayoub AM, et al. Inhibiting APOBEC3 activity with single-stranded DNA containing 2’-deoxyzebularine analogues. Biochemistry. 2019;58(5):391–400. doi: 10.1021/acs.biochem.8b00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.King JJ, Borzooee F, Im J, Asgharpour M, Ghorbani A, Diamond CP, Fifield H, Berghuis L, Larijani M. Structure-Based Design of First-Generation Small Molecule Inhibitors Targeting the Catalytic Pockets of AID, APOBEC3A, and APOBEC3B. CS Pharmacol. Transl. Sci. 2021;4(4):1390–1407. doi: 10.1021/acsptsci.1c00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mimura K, Teh JL, Okayama H, Shiraishi K, Kua LF, Koh V, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109(1):43–53. doi: 10.1111/cas.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Thiem A, Hesbacher S, Kneitz H, di Primio T, Heppt M, Hermanns HM, et al. IFN-gamma-induced PD-L1 expression in melanoma depends on p53 expression. J Exp Clin Cancer Res. 2019;38(1):397. doi: 10.1186/s13046-019-1403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vile RG, Melcher A, Pandha H, Harrington KJ, Pulido JS. APOBEC and cancer viroimmunotherapy: thinking the unthinkable. Clinical Cancer Res. 2021;27(12):3280–3290. doi: 10.1158/1078-0432.CCR-20-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Serebrenik AA, Starrett GJ, Leenen S, Jarvis MC, Shaban NM, Salamango DJ, et al. The deaminase APOBEC3B triggers the death of cells lacking uracil DNA glycosylase. Proc Natl Acad Sci. 2019;116(44):22158–22163. doi: 10.1073/pnas.1904024116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Biayna J, Garcia-Cao I, Álvarez MM, Salvadores M, Espinosa-Carrasco J, McCullough M, et al. Loss of the abasic site sensor HMCES is synthetic lethal with the activity of the APOBEC3A cytosine deaminase in cancer cells. PLoS Biol. 2021;19(3):e3001176. doi: 10.1371/journal.pbio.3001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.