Figure 2.
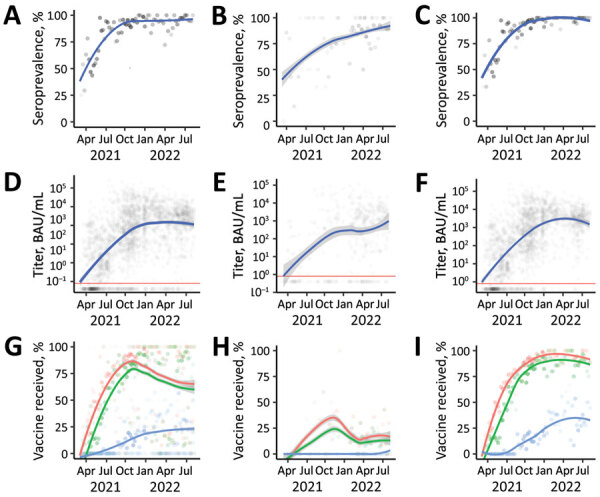
SARS-CoV-2 S antibody seroprevalence, titers, and vaccine doses of participants enrolled (N = 2,300) in a study of SARS-CoV-2 S antibody levels, by age group, Dominican Republic, March 2021–May 2022. A–C) Seroprevalence among study participants of all ages (A), 2–17 years of age (B), and >18 years of age (C). Gray dots indicate weekly mean values; increased dot intensity reflected more observations. Blue line indicates locally estimated scatterplot smoothing (LOESS) smoothed seroprevalence; gray shading indicates 95% CI around the smoothed estimate. D–F) Titers among study participants of all ages (D), 2–17 years of age (E), and >18 years of age (F), by week, plotted on a log scale. Each gray dot indicates a unique study participant (n = 1,910). Blue lines indicate LOESS smoothed antibody levels; gray shading indicates 95% CI around the smoothed estimate. Horizontal red line indicates manufacturer recommended cutoff index (>0.800 BAU /mL); values above the line represent a positive result and values below the line a negative result. G–I) Percentage of weekly enrolled participants of all ages (G), 2–17 years of age (H), and >18 years of age (I) who had received >1 (red dots), >2 (green dots), or >3 (blue dots) COVID-19 vaccine doses; increased dot intensity reflects more observations. Colored lines indicate LOESS smoothed percentage; gray shading indicates 95% CI around smoothed percentage. BAU, binding antibody units; S, spike.
