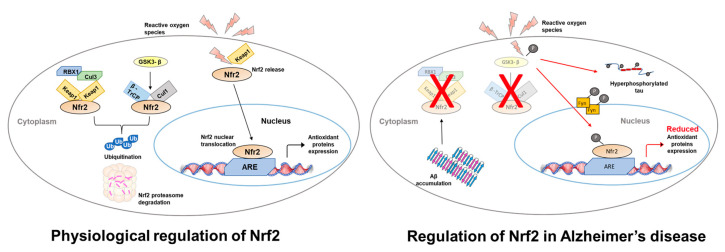
Figure 1.
Schematic representation of Nrf2 regulation in neurons. Under physiological conditions Nrf2 signaling is regulated in two ways: (i) Keap 1 sequesters Nrf2, which leads to Nrf2-ubiquitination through the formation of the Keap1–Cul3-Rbx1 complex. Nrf2 is then targeted to and degraded by the proteasome; (ii) GSK-3β phosphorylates Nrf2, which then binds to an E3 ligase and β-TrCP. This allows the ubiquitin-proteasome degradation of Nrf2. High ROS levels lead to positive regulation of the Nrf2 pathway by releasing Nrf2 from Keap1. Nrf2 is then free to translocate into the nucleus where it binds ARE gene sequences activating their expression. In AD, Aβ42 accumulation (i) increases ROS production and (ii) blocks the regulation of Nrf2 by stabilizing the interaction between Keap1 and Nrf2. This ultimately leads to a reduced expression of the oxidative stress response genes, which further leads to an increase in ROS levels. Moreover, GSK-3β, which is involved in tau phosphorylation, contributes to Nrf2 degradation by proteasome via a Fyn-mediated mechanism.