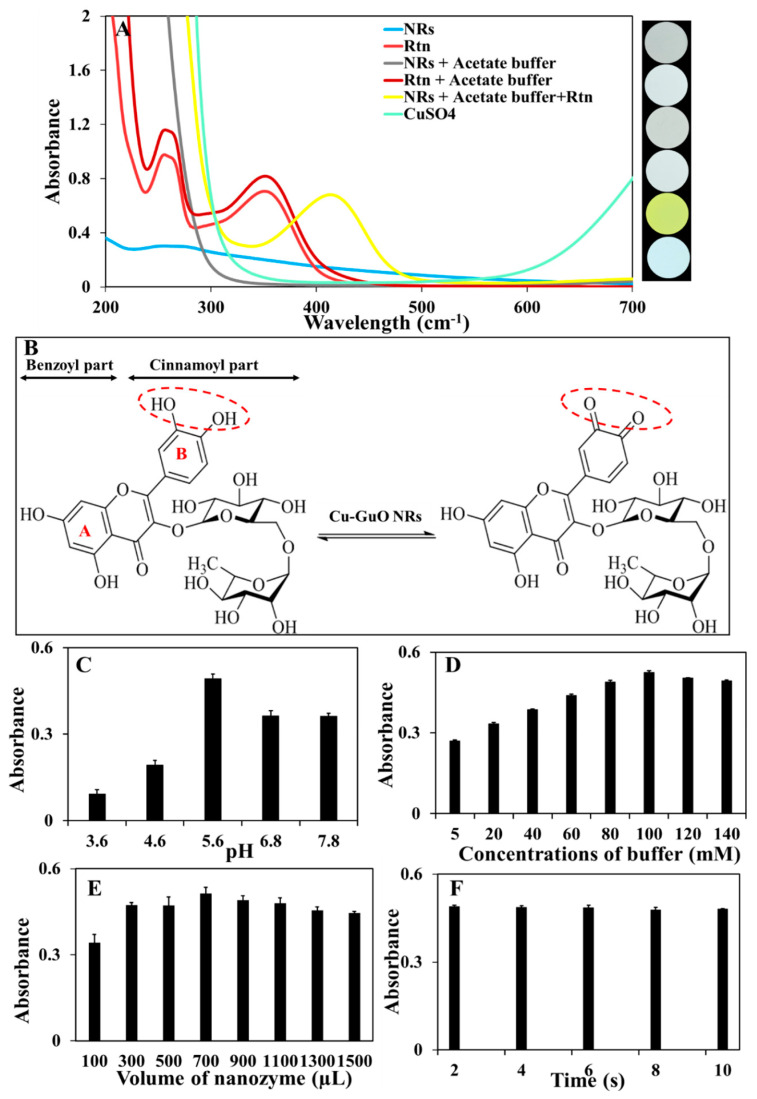
Figure 3.
(A) UV–Vis spectra of the different solutions: 700 µL of Cu-Guo NRs (1 mg m), Rtn (46.68 μM), 700 µL of Cu-Guo NRs (1 mg m) in the presence of acetate buffer (100 mM, pH of 5.6); Rtn (46.68 μM) in the presence of acetate buffer (100 mM, pH of 5.6); Rtn (46.68 μM) in the presence of acetate buffer (100 mM, pH of 5.6); and 700 µL of NRs (1 mg m) and CuSO4 (800 μM) with their corresponding images (from top to down). (B) The proposed possible sensing strategy of Rtn based on the catalytic oxidation reaction by Cu-Guo NRs. The effect of the (C) buffer type in a solution containing acetate buffer (100 mM) with different pH values of 3.6, 4.6, and 5.6 and HEPES buffer (100 mM) with different pH values from 6.8 to 7.8, in the presence of Rtn (46.68 µM) and 700 µL of Cu-Guo NRs (1 mg m). (D) Buffer concentration in a solution containing acetate buffer (pH of 5.6) with different concentrations from 5 mM to 140 mM, Rtn (46.68 μM) and 700 µL of Cu-Guo NRs (1 mg m). (E) Nanozyme volume in a solution containing 100 mM of acetate buffer (pH of 5.6) and various volumes of Cu-Guo NRs from 100 µL to 1500 µL and Rtn (46.68 μM). (F) Reaction time from 2 min to 10 min for a solution containing 100 mM of acetate buffer (pH of 5.6) and Rtn (46.68 µM) and 700 µL of Cu-Guo NRs (1 mg m).