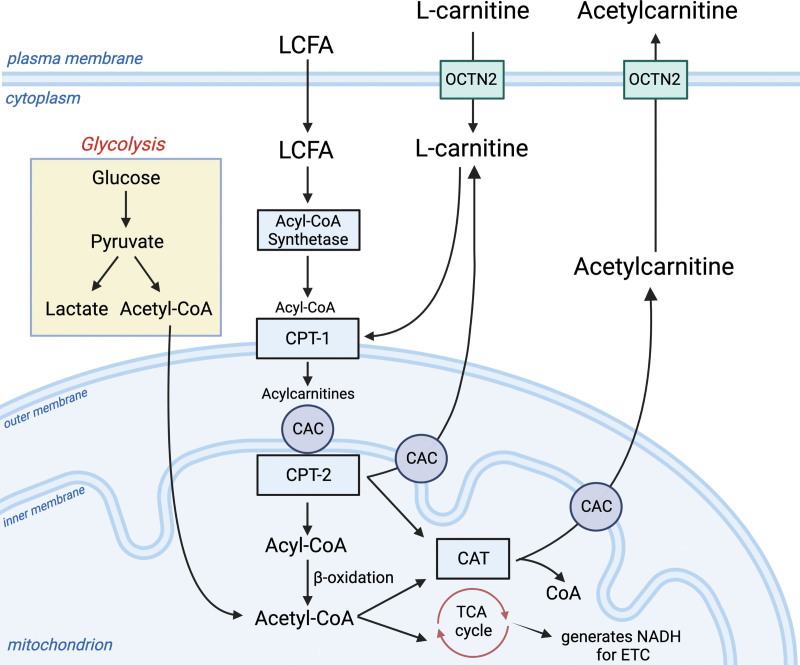
Figure 1.
Glycolysis and mitochondrial-related metabolism. L-carnitine is required for the transport of long-chain fatty acids (LCFA) into the mitochondrion. This process enlists the carnitine shuttle, which begins with the entrance of L-carnitine into the cell from the blood through the organic cation transporter (OCTN2). LCFAs enter the cell and are converted to long-chain acyl-CoAs by acyl-CoA synthetase (EC 6.2.1.3). The L-carnitine shuttle apparatus uses L-carnitine via carnitine palmitoyl transferase 1 (CPT1; EC 2.3.1.21) to convert L-carnitine and LCFA-CoAs to acylcarnitines. The transporter, carnitine-acylcarnitine carrier (CAC; SLC25A20), moves the newly formed long-chain acylcarnitines into the mitochondrial matrix in exchange for free carnitine. Here, long-chain acyl groups are transferred back to CoA by CPT2 (EC 2.3.1.21), which regenerates Acyl-CoA for use in β-oxidation (sepsis-induced dysfunction of β-oxidation can lead to elevations in acylcarnitines). This process also generates L-carnitine. Here, L-carnitine can either be transported out of the mitochondrion by CAC or used as a substrate for carnitine acetyl-transferase (CAT; EC2.3.1.7), which converts it and Acetyl-CoA to acetylcarnitine. Acetylcarnitine moves through CAC and OCTN2 back into the bloodstream. This process may be enhanced during sepsis and times of metabolic stress, serving as a crucial sink for excess acetyl groups that may be toxic to the cell. The Acetyl-CoA produced by β-oxidation sources the tricarboxylic acid (TCA) cycle as does the Acetyl-CoA produced from the oxidative decarboxylation of pyruvate in the mitochondrial matrix. NADH = nicotinamide adenine dinucleotide (NAD) + hydrogen (H); CoA = coenzyme A; ETC = electron transport chain. This figure was created using Biorender.com.