Abstract
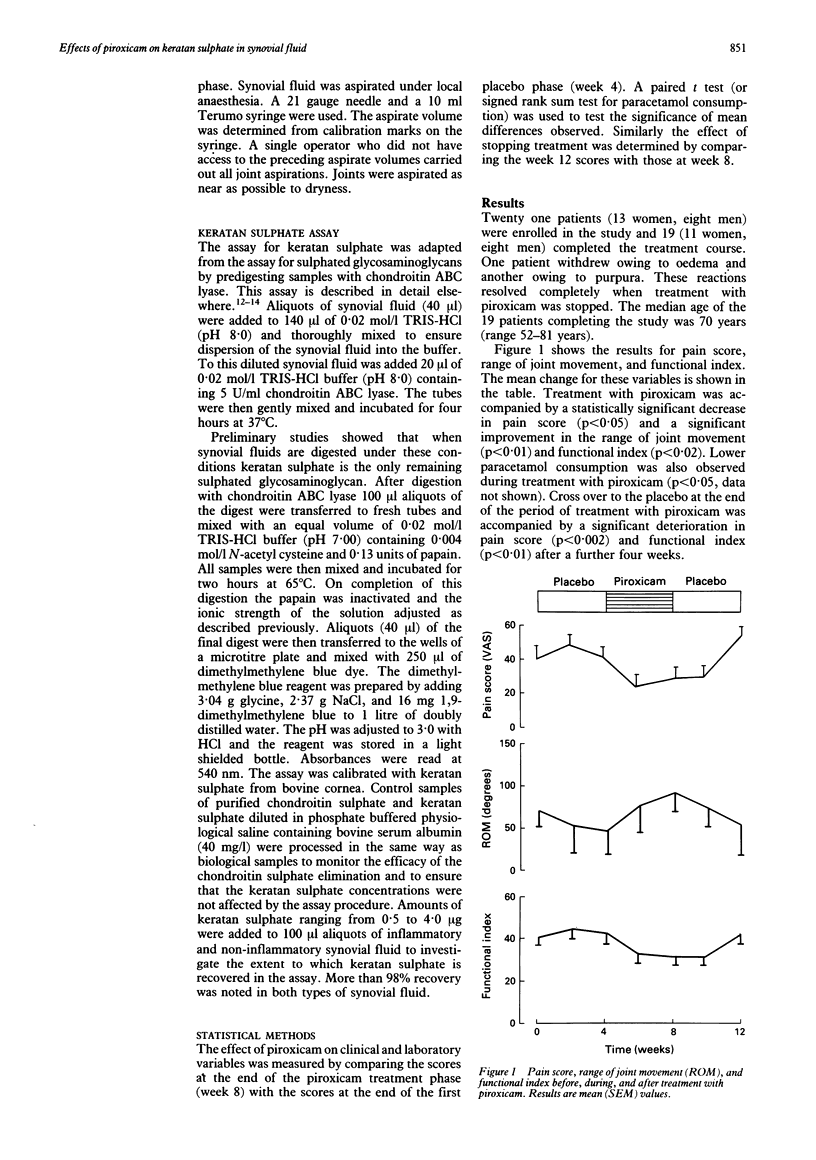
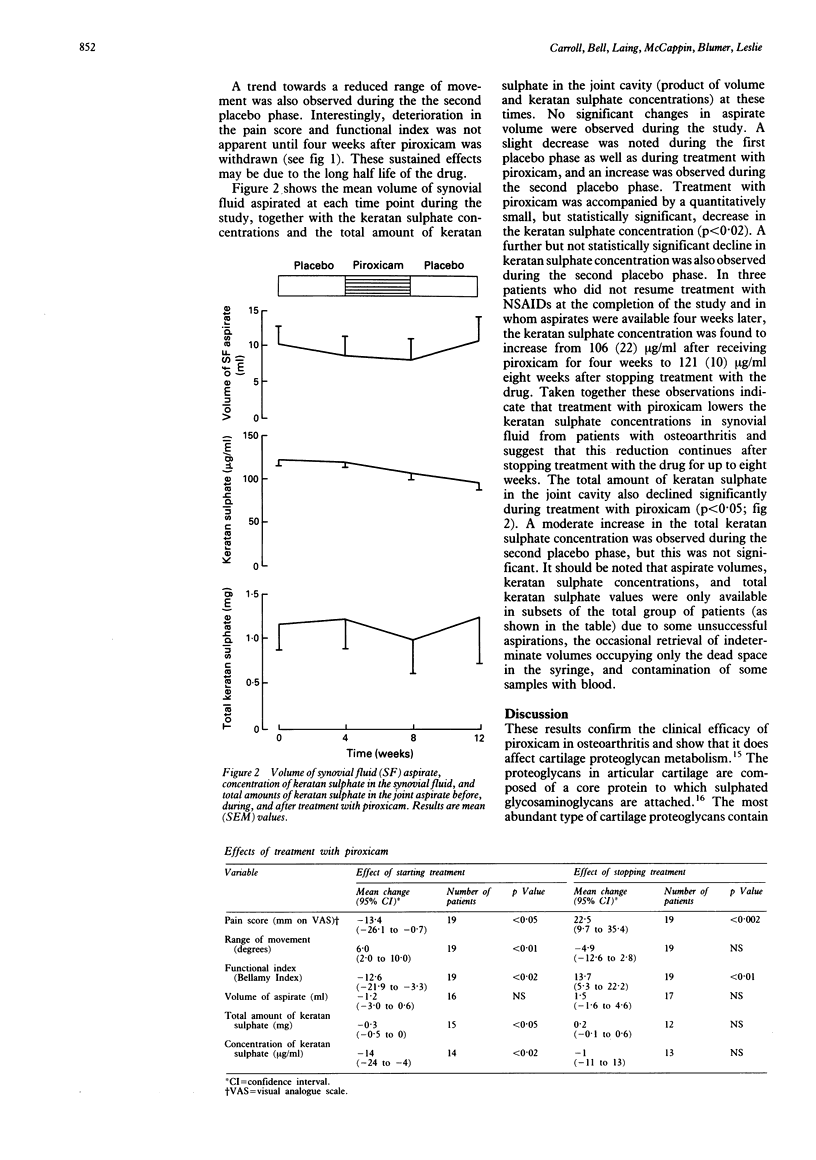
To study the effects of piroxicam on cartilage metabolism in vivo, a three phase (placebo/piroxicam 20 mg/day by mouth/placebo) double blind controlled trial was conducted in patients with osteoarthritis of the knee joint. Twenty one patients were recruited, 19 of whom (11 women, eight men, median age 70 years) completed the treatment schedule. The knee joint under study was aspirated to dryness at four week intervals. Treatment with piroxicam was accompanied by a decrease in the pain score, an improvement in the functional index, and an increased range of movement. Reductions in the concentration (mean (SEM) 120 (6) to 110 (8) micrograms/ml) and the total amount (1.22 (0.34) to 0.99 (0.37) mg) of keratan sulphate, but not the effusion volume (9.4 (2.5) to 8.3 (2.6) ml) were observed during treatment with piroxicam. These findings are consistent with decreased proteoglycan catabolism during treatment with piroxicam. Neither depressed synthesis nor enhanced clearance of degraded proteoglycan fragments can be excluded, however.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Bellamy N., Buchanan W. W. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986 Jun;5(2):231–241. doi: 10.1007/BF02032362. [DOI] [PubMed] [Google Scholar]
- Carroll G. J. Spectrophotometric measurement of proteoglycans in osteoarthritic synovial fluid. Ann Rheum Dis. 1987 May;46(5):375–379. doi: 10.1136/ard.46.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll G., McCappin S., Bell M., Schwarzer A., Breidahl P. Comparison of keratan sulphate concentrations and the size distribution of proteoglycans in the synovial fluid of patients with osteoarthritis and pyrophosphate arthropathy. Rheumatol Int. 1991;11(2):63–68. doi: 10.1007/BF00291147. [DOI] [PubMed] [Google Scholar]
- Habuchi H., Yamagata T., Iwata H., Suzuki S. The occurrence of a wide variety of dermatan sulfate-chondroitin sulfate copolymers in fibrous cartilage. J Biol Chem. 1973 Sep 10;248(17):6019–6028. [PubMed] [Google Scholar]
- Hamerman D., Smith C., Keiser H. D., Craig R. Glycosaminoglycans produced by human synovial cell cultures. Coll Relat Res. 1982 Jul;2(4):313–329. doi: 10.1016/s0174-173x(82)80023-x. [DOI] [PubMed] [Google Scholar]
- Page-Thomas D. P., Bard D., King B., Dingle J. T. Clearance of proteoglycan from joint cavities. Ann Rheum Dis. 1987 Dec;46(12):934–937. doi: 10.1136/ard.46.12.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Aspirin aggravates the degeneration of canine joint cartilage caused by immobilization. Arthritis Rheum. 1982 Nov;25(11):1333–1342. doi: 10.1002/art.1780251109. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Effect of salicylate on proteoglycan metabolism in normal canine articular cartilage in vitro. Arthritis Rheum. 1979 Jul;22(7):746–754. doi: 10.1002/art.1780220710. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Effects of some nonsteroidal antiinflammatory drugs on proteoglycan metabolism and organization in canine articular cartilage. Arthritis Rheum. 1980 Sep;23(9):1010–1020. doi: 10.1002/art.1780230908. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. In vivo effect of aspirin on canine osteoarthritic cartilage. Arthritis Rheum. 1983 Aug;26(8):994–1001. doi: 10.1002/art.1780260808. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Colyer R. A., Brandt K. D. Marked suppression by salicylate of the augmented proteoglycan synthesis in osteoarthritic cartilage. Arthritis Rheum. 1980 Jan;23(1):83–91. doi: 10.1002/art.1780230114. [DOI] [PubMed] [Google Scholar]
- SENO N., MEYER K., ANDERSON B., HOFFMAN P. VARIATIONS IN KERATOSULFATES. J Biol Chem. 1965 Mar;240:1005–1010. [PubMed] [Google Scholar]
- Verbruggen G., Veys E. M., Malfait A. M., Cochez P., Schatteman L., Wieme N., Heynen G., Broddelez C. Proteoglycan metabolism in tissue cultured human articular cartilage. Influence of piroxicam. J Rheumatol. 1989 Mar;16(3):355–362. [PubMed] [Google Scholar]
- Verbruggen G., Veys E. M., Malfait A. M., Schatteman L., Wieme N., Heynen G., Vanhoutte V., Broddelez C. Proteoglycan metabolism in isolated chondrocytes from human cartilage and in short-term tissue-cultured human articular cartilage. Clin Exp Rheumatol. 1989 Jan-Feb;7(1):13–17. [PubMed] [Google Scholar]
- Wallis W. J., Simkin P. A., Nelp W. B., Foster D. M. Intraarticular volume and clearance in human synovial effusions. Arthritis Rheum. 1985 Apr;28(4):441–449. doi: 10.1002/art.1780280413. [DOI] [PubMed] [Google Scholar]


