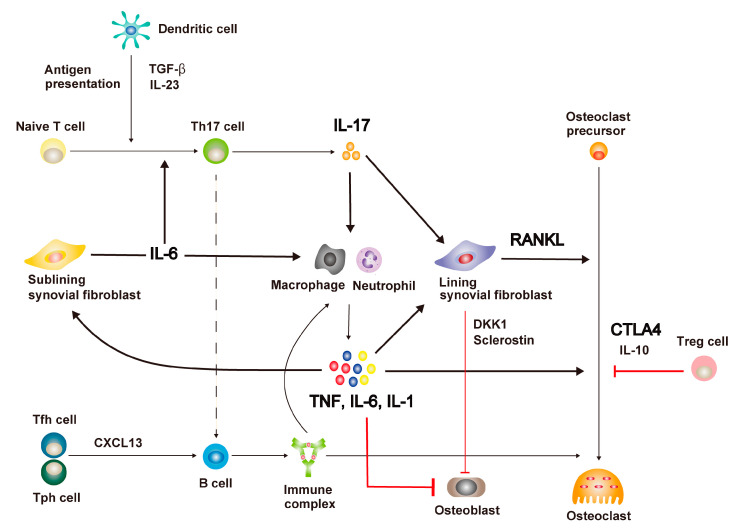
Figure 1.
The pathway of inflammation and bone destruction in rheumatoid arthritis and the mechanism of action of biological agents. In rheumatoid arthritis (RA) synovium, antigen-presenting cells (APCs), including dendritic cells, present self-antigens and produce transforming growth factor-β (TGF-β) and interleukin-23 (IL-23), which promote T helper 17 cell (Th17) differentiation and IL-17 secretion. IL-17 stimulates the production of pro-inflammatory cytokines, such as tumor necrosis factor (TNF), IL-6, and IL-1, by synovial macrophages and neutrophils, and induces receptor activator of NF-κB ligand (RANKL) expression in lining synovial fibroblasts. Pro-inflammatory cytokines further promote IL-6 production by the sub-lining synovial fibroblasts and RANKL expression in the lining of synovial fibroblasts and activate the osteoclast differentiation pathway. Immune complexes produced by B cells, upon stimulation of chemokine (C-X-C motif) ligand 13 (CXCL13) from T follicular helper (Tfh) and T peripheral helper (Tph) cells, boost local inflammation and osteoclast differentiation. Dickkopf-related protein 1 (DKK1) and sclerostin, produced by the lining synovial fibroblasts, and pro-inflammatory cytokines inhibit osteoblast differentiation. Anti-IL-17 antibodies suppress inflammation and bone destruction. Anti-TNF antibodies and anti-IL-1 antibodies inhibit inflammation and bone destruction, simultaneously improving bone repair capacity. Anti-IL-6 antibodies restrain inflammation, bone destruction, and Th17 cell differentiation besides contributing to the improvement in bone repair capability. CTLA4 immunoglobulin prevents osteoclast differentiation. Anti-RANKL antibody directly inhibits bone destruction.