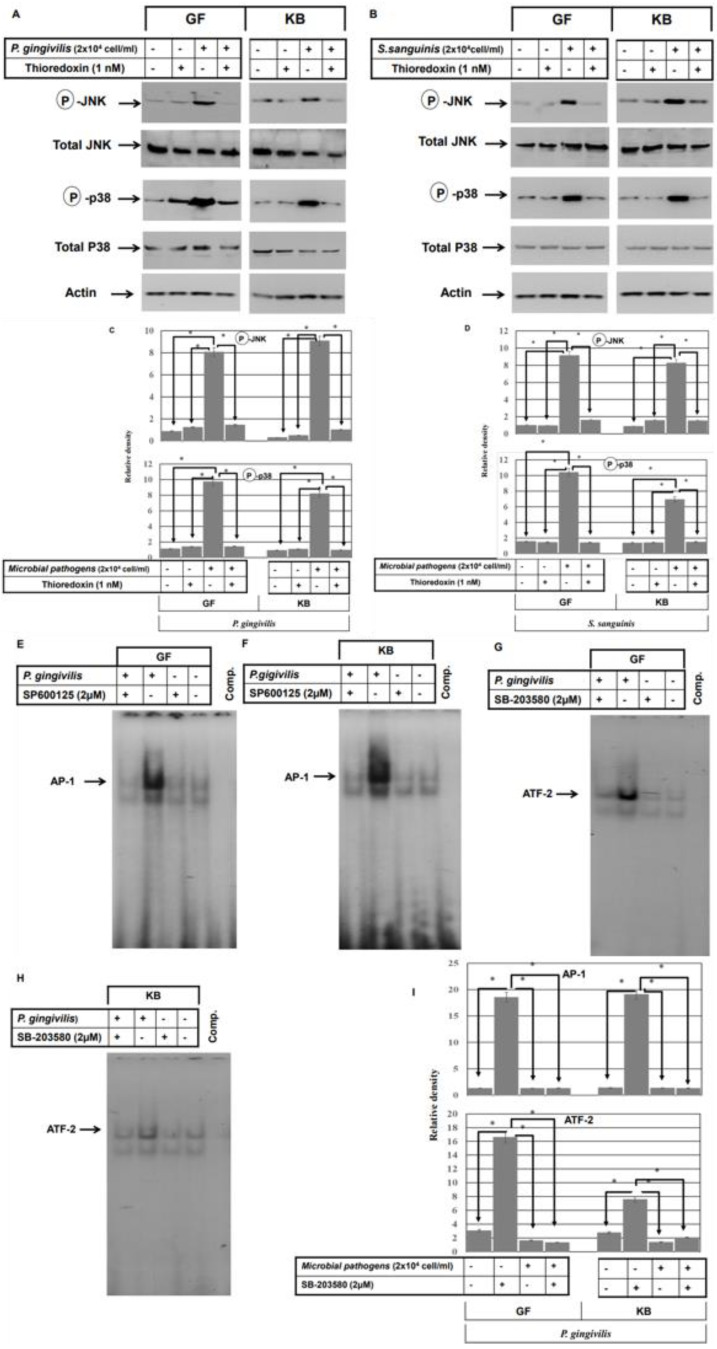
Figure 4.
ASK1 mediates the activation of both the JNK and p38 pathways in oral mucosa by stimulation with bacterial pathogens. (A–F) Both GF and KB cells were pre-treated with the inhibitor of ASK1 (thioredoxin) 1 h prior to stimulation with either heat-inactivated P. gingivalis (2 × 104 cells/mL) or heat-activated S. sanguinis (2 × 104 cells/mL) microbial pathogens (MPs) for 48 h. Total protein lysate and nuclear extract were then prepared for Western blot analysis and EMSA assay. (A,B) Western blot analysis of the total lysate of treated and control cells using anti-phospho-JNK, phospho-p38, JNK, or p38 antibodies demonstrate the inhibition of inactivated P. gingivalis (A) and S. sanguinis (B)-induced phosphorylation of both JNK and p38 proteins by the inhibitor of ASK1 in both GF and KB cells when compared with control cells. JNK, p38, and actin were used as internal controls for loading and transfer. (C,D) Analysis of band intensity on films is presented as the relative ratio of Phospho-JNK and Phospho-p38 to the expression of JNK and p38 expression. Bars represent the mean ± SD from three independent experiments performed separately, * p < 0.05. (E,F) Pre-treatment of both GF and KB cells with ASK1 inhibitor suppressed the induced DNA-binding activity of the transcription factors AP-1 (C,D) and ATF-2 (E,H) by the stimulation of both GF and KB cells with either heat-inactivated P. gingivalis (E,G) or heat-inactivated S. sanguinis (F,H) bacterial pathogens. (I) Analysis of band intensity of the transcription factors AP-1 and ATF-2. Bars represent mean ± SD from three independent experiments performed separately, * p < 0.05.