Figure 1.
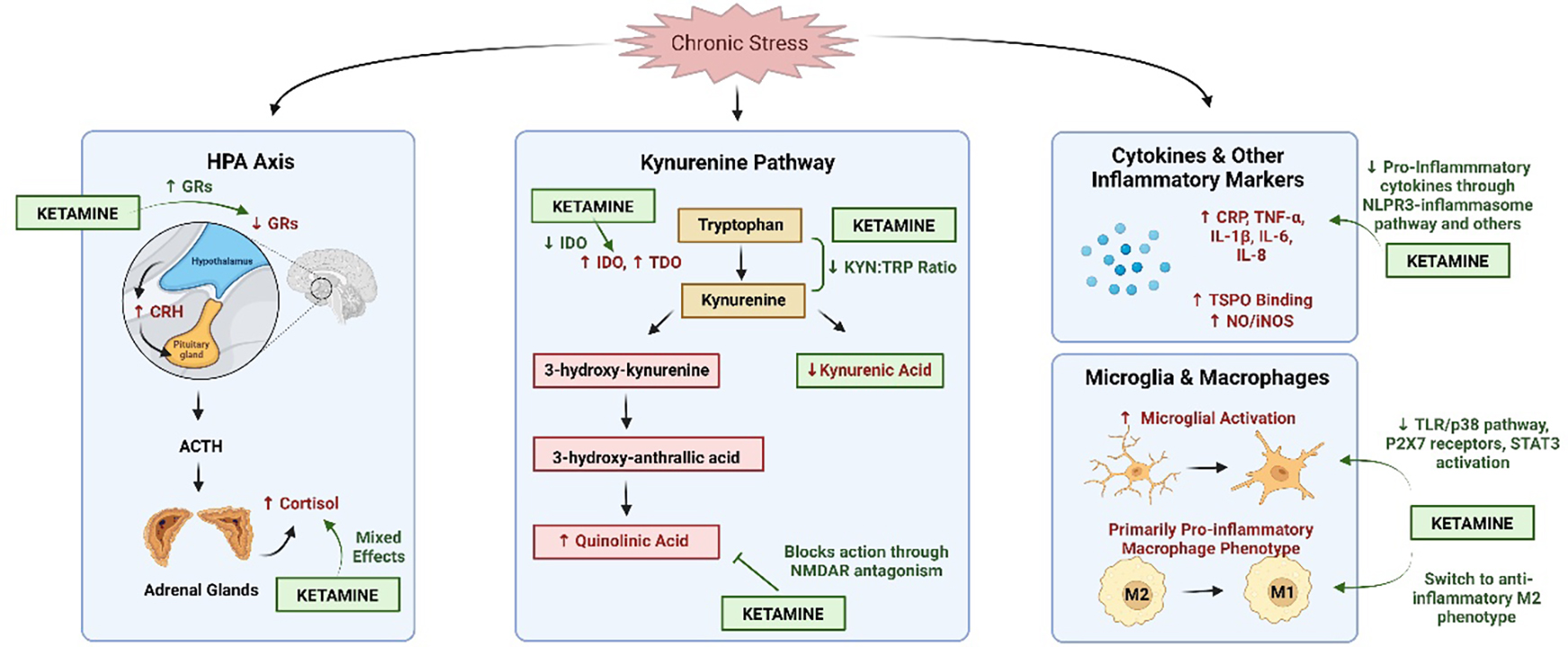
The hypothesized impact of ketamine on stress and inflammatory pathways. Chronic stress leads to overactivation of the hypothalamic-pituitary-adrenal (HPA) axis, which increases levels of corticotropin-releasing hormone (CRH) and cortisol while decreasing expression of glucocorticoid receptors (GRs). This decrease in GR expression prevents the shut-off of the HPA axis, leading to prolonged activation that can have negative consequences. Ketamine, an N-methyl-d-aspartate receptor (NMDAR) antagonist, appears to mediate this stress response by increasing the number of GRs. Studies examining ketamine’s effect on cortisol levels have yielded mixed results. Under chronic stress conditions, the kynurenine pathway, another potential mediator between stress and inflammation, demonstrates increased levels of indoleamine-2,3-dioxygenase (IDO), tryptophan-2,3-dioxygenase (TDO) and quinolinic acid, as well as decreased levels of kynurenic acid. Ketamine decreases IDO levels and the ratio of kynurenine:tryptophan through indirect mechanisms while blocking the action of quinolinic acid through direct NMDAR antagonism. Ketamine also decreases proinflammatory cytokine levels (increased by chronic stress) through the NLPR3-inflammasome pathway, decreasing microglial activation via TLR/p38 signaling, P2X7 receptors and signal transducer and activator of transcription 3 (STAT3) activation, as well as switching macrophages to the anti-inflammatory M2 phenotype. Figure created using Biorender.
