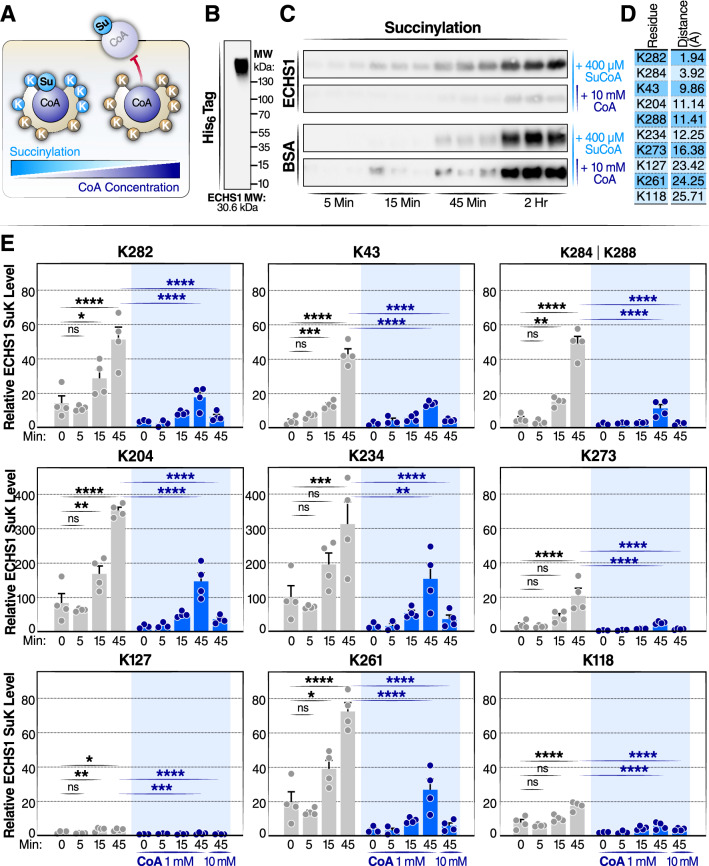
Figure 5.
In-vitro succinylation of ECHS1 with succinyl-CoA is competitively inhibited by CoA (a) Schematic diagram of the succinylation of ECHS1 by succinyl-CoA (Su-CoA) and inhibition by CoA. (b) Western blot of human recombinant ECHS1 using native gel electrophoresis. (c) Western blot detection of succinyl lysine (SuK) levels in ECHS1 and BSA co-incubated with Su-CoA in the presence and absence of CoA for varying times (5, 15, and 45 min, and 2 h). (d) Distance of individual lysine residues to CoA on ECHS1. (e) Mass spectrometry time-course experiment measuring the change in succinylation levels at individual lysines on ECHS1. ECHS1 was co-incubated with 400 µM Su-CoA for 0, 5, 15 or 45 min and with 0, 1 or 10 mM CoA. The abundance of SuK levels for each residue was normalized to the ECHS1 negative control (not treated with CoA or Su-CoA), (N = 4 per treatment). *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001; ns, not significant. A one-way ANOVA was performed. In the conditions not treated with CoA, each time point was compared to the 0 min group. In the 45-min conditions, both CoA-treated groups (1 and 10 mM) were compared to the group not treated with CoA.