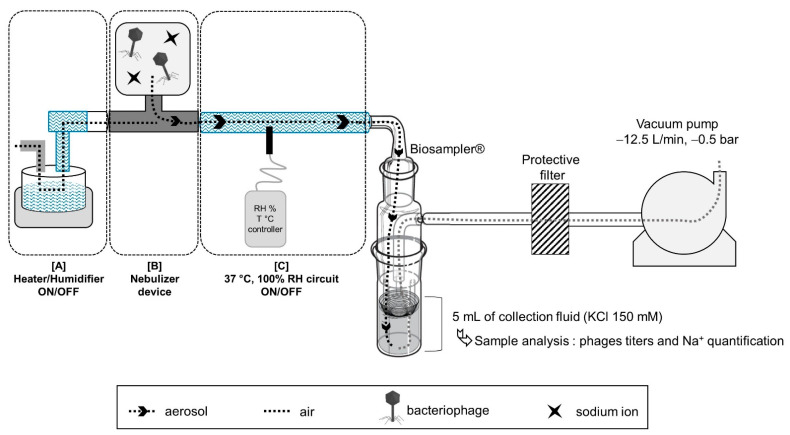
Figure 2.
Bench study for in vitro assessment of parameters influencing phage viability: phage morphotypes and humidity. In the center of the figure, the BioSampler® gently aspirates and collects the aerosol containing the bacteriophage and sodium ion from the top opening through a 5 mL vortex fluid of KCl (150 mM), by following −0.5 bar depressurized air from a vacuum pump settled at −12.5 L/min. Step one was to connect the nebulizer device alone [B] to the BioSampler® to assess the impact of different nebulizers on bacteriophage viability. Step 2 [A] + [B] + [C] was to aerosolize bacteriophages with a specific nebulizer device connected to a heated and humidified circuit. For the two steps, after aerosol collection, samples were analyzed to quantify bacteriophage titers using a soft-agar overlay method (infective phage titration) and sodium ions using ICP-MS (output rate of aerosol collection).