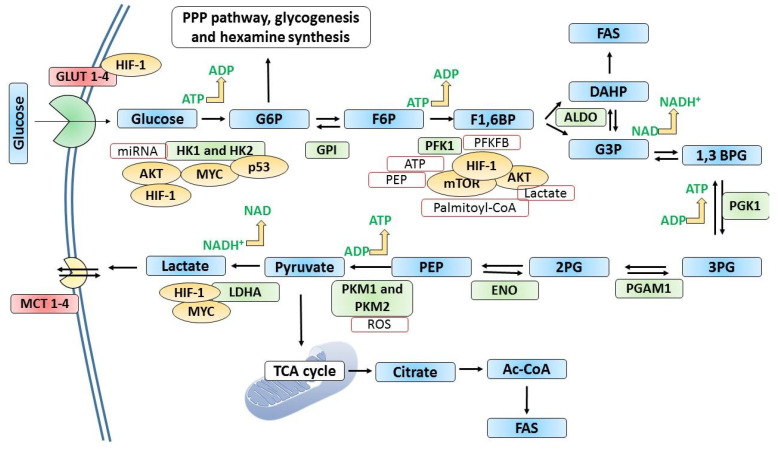
Figure 3.
The metabolism of glucose in cancer cells. In many cancer types, glucose uptake and glycolysis occur at faster rates compared to normal cells. When the oxygen supply or levels are scarce, pyruvate generated by glycolysis is diverted away from mitochondrial oxidative phosphorylation by producing lactate (anaerobic glycolysis). Otto Warburg observed that glycolysis occurs at a higher rate in cancer cells despite the presence of oxygen, converting more glucose to lactate. This “Warburg” effect is the fundamental basis for detecting and treating several human tumors. For example, the phosphorylated form of [18F]2-fluoro-2-deoxy glucose (FdG) is not converted in the GPI-catalyzed reaction in glycolysis, where its accumulation is detected using positron emission tomography. Glucose transporters and many glycolytic enzymes are overexpressed or overproduced in cancer cells. Hypoxia-inducible factor-1 (HIF-1) induces or regulates the expression of key glycolytic enzymes and GLUTs. GLUT1 and GLUT3 are frequently overexpressed in cancer. Tumor suppressor p53 and some oncoproteins such as AKT, MYC, and mTOR regulate the expression of key glycolytic enzymes as shown in the figure. In addition, metabolites such as ROS, ATP, PEP, lactate, and palmitoyl-CoA exert regulatory effects as indicated. 1,3 BPG; 1,3-bisphosphoglycerate, 2PG; 2-phosphoglycerate, 3PG; 2-phosphoglycerate, ALDO; aldolase, DAHP; dihydroxyacetone phosphate, ENO; enolase, F1,6BP; fructose-1,6-bisphosphate, F6P; fructose-6-phosphate, FAS; fatty acid synthesis, G3P; glyceraldehyde 3-phosphate, G6P; glucose 6 phosphate, GLUTs; glucose transporters, GPI; glucose-6-phosphate isomerase, HK1 and HK2; hexokinase 1 and 2; LDHA; lactate dehydrogenase, MCT; monocarboxylate transporters, PEP; phosphoenolpyruvate, PFK1; phosphofructokinase-1, PFKFB; 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase, PGAM1; phosphoglycerate mutase 1, PGK1; phosphoglycerate kinase 1, PKM1; pyruvate kinase 1 and PKM2; pyruvate kinase 2.