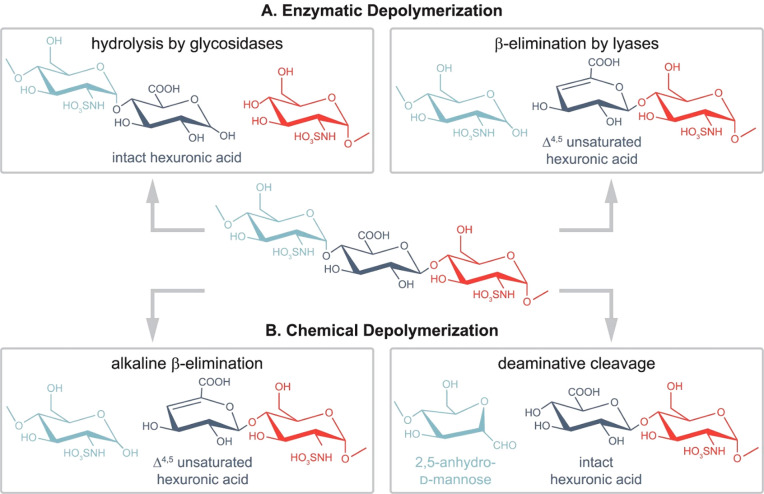
Figure 2.
Common glycosaminoglycan depolymerization strategies are illustrated through the example of heparan sulfate/heparin. (A, left) When glycosidases are used to depolymerize GAG chains, the resulting cleavage preserves the hexuronic acid stereochemistry. Enzymes with endolytic activity are required to obtain oligosaccharides covering the full sequence. As for moderately sulfated HS/heparin chains, using heparanase as endo β-glucuronidases cleaves at the reducing end of GlcA residues (A, right). Prokaryotic lyases, such as heparinase I–III, follow a β-eliminative mechanism, resulting in Δ4,5-unsaturated uronic acid residues at the new nonreducing end. Consequently, stereochemical information is lost in the process (B, left). Benzyl esterification with alkaline β-elimination mimics lyase activity and creates Δ4,5-unsaturated uronic acid residues at the new nonreducing end (B, right). While preserving hexuronic acid stereochemical information at the cleavage site, deamination cleavage alters the structure of the glucosamine through the formation of 2,5-anhydromannose. N-Acetyl groups on glucosamines block the reaction, making prior deacetylation necessary. Reproduced from ref (3). Copyright 2022, American Chemical Society.