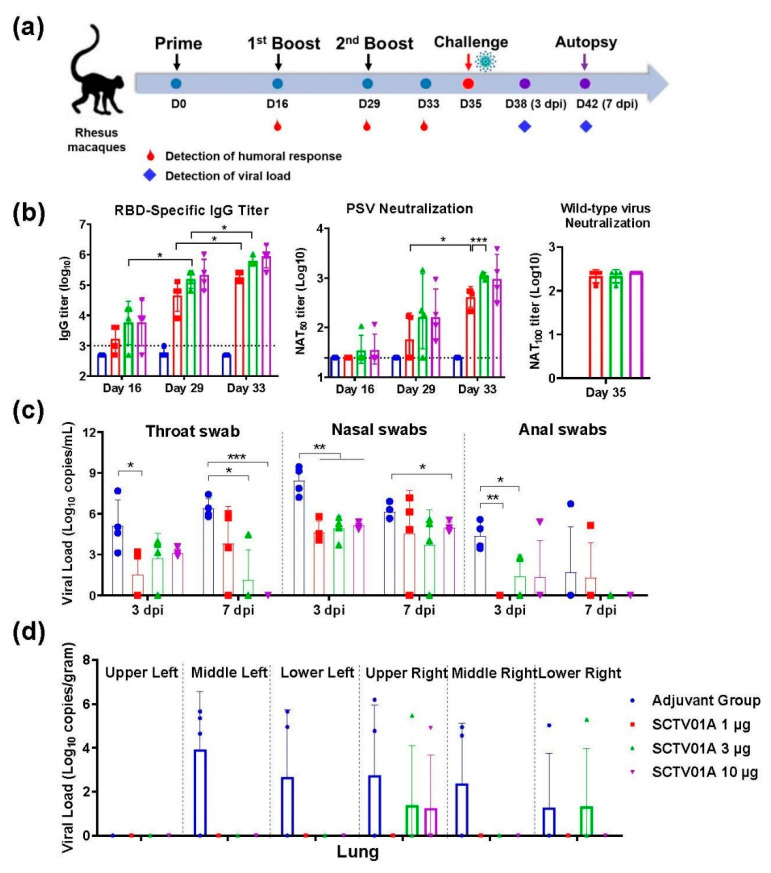
Figure 3.
Immunogenicity and protective efficacy of SCTV01A in rhesus macaques. (a) Immunization and challenge procedures for rhesus macaques. Rhesus macaques (n = 4/group) were immunized with 1 μg, 3 μg, or 10 μg SCTV01A/SCT-VA02B on day 0, 16, and 29, respectively. The control group was treated with an equal volume of SCT-VA02B. The SARS-CoV-2 (Strain: GD108#) challenge was conducted on day 35. Swabs of throat, nasal, and anal were collected at 3 and 7 dpi. Macaques were sacrificed at 7 dpi. Tissues, such as lungs, heart, and liver, were collected. (b) RBD-specific IgG titer (left) and PsV NAT50 titer (middle) at the indicated time points post-immunization. SARS-CoV-2 authentic virus neutralizing titer NAT100 at Day35 (right). (c) Viral load of throat, nasal, and anal swabs at 3 dpi and 7 dpi. (d) SARS-CoV-2 viral load was determined using RT-qPCR at 7 dpi in lungs. Statistical analyses were performed using unpaired two-tailed Welch’s tests. * p < 0.05, ** p < 0.001, *** p < 0.0001.