Abstract
Aims
The Iron Intravenous Therapy in Reducing the burden of Severe Arrhythmias in HFrEF (RESAFE‐HF) registry study aims to provide real‐word evidence on the impact of intravenous ferric carboxymaltose (FCM) on the arrhythmic burden of patients with heart failure with reduced ejection fraction (HFrEF), iron deficiency (ID), and implanted cardiac implantable electronic devices (CIEDs).
Methods and results
The RESAFE‐HF (NCT04974021) study was designed as a prospective, single‐centre, and open‐label registry study with baseline, 3, 6, and 12 month visits. Adult patients with HFrEF and CIEDs scheduled to receive IV FCM as treatment for ID as part of clinical practice were eligible to participate. The primary endpoint is the composite iron‐related endpoint of haemoglobin ≥ 12 g/dL, ferritin ≥ 50 ng/L, and transferrin saturation > 20%. Secondary endpoints include unplanned HF‐related hospitalizations, ventricular tachyarrhythmias detected by CIEDs and Holter monitors, echocardiographic markers, functional status (VO2 max and 6 min walk test), blood biomarkers, and quality of life. In total, 106 patients with a median age of 72 years (14.4) were included. The majority were male (84.9%), whereas 92.5% of patients were categorized to New York Heart Association II/III. Patients' arrhythmic burden prior to FCM administration was significant—19 patients (17.9%) received appropriate CIED therapy for termination of ventricular tachyarrhythmia in the preceding 12 months, and 75.5% of patients have frequent, repetitive multiform premature ventricular contractions.
Conclusions
The RESAFE‐HF trial is expected to provide evidence on the effect of treating ID with FCM in HFrEF based on real‐world data. Special focus will be given on the arrhythmic burden post‐FCM administration.
Keywords: Heart failure, HFrEF, Iron deficiency, Arrhythmia, Ferric carboxymaltose, FCM
Introduction
Heart failure (HF) is a common chronic disease, affecting 11.8% of people aged over 65 in developed countries. 1 HF constitutes a sizeable and ever‐expanding burden on healthcare systems. 2 Its effect on patients cannot be understated; functional capacity and quality of life are greatly impaired, 3 whereas the odds of 5 year survival after diagnosis is only 57% in recent registry cohorts. 4
Sudden cardiac death (SCD) secondary to ventricular tachyarrhythmias is a major cause of mortality in heart failure with reduced ejection fraction (HFrEF), 4 especially in patients who are in earlier stages of the disease. Cardiac implantable electronic devices (CIEDs) with cardioversion–defibrillation technology [implantable cardioverter–defibrillator (ICD)] are a mainstay in SCD prevention in HFrEF. These devices have greatly improved over time and are capable to monitor and record patients' arrhythmic events, delivering life‐saving therapy when deemed necessary. Nonetheless, these devices are neither curative nor 100% successful in combating lethal arrhythmias. 5
Indeed, the pathophysiology of ventricular arrhythmias in HFrEF is complex and not entirely clear; several factors from neurohormonal activation to ventricular dilation and fibrosis have been implicated in arrhythmogenesis. Irrespective of potential precipitating factors, electrochemical instability at the cellular level represents an ideal and almost essential substrate for arrhythmogenesis. 6
Iron deficiency (ID) is present in more than half of HF patients 7 , 8 and correlates with increased mortality independent of other factors, including anaemia. 9 Evidence from multiple 10 , 11 , 12 , 13 double‐blind, randomized controlled trials indicates that iron supplementation with intravenous ferric carboxymaltose (FCM) reverses ID reliably in HFrEF patients, thereby significantly reducing the risk of hospitalization and improving functional capacity and quality of life. Based on these data, the 2016 European Society of Cardiology (ESC) guidelines 14 recommend considering intravenous FCM in symptomatic HFrEF patients with ID to improve symptoms, exercise capacity, and quality of life. The newly presented version of the guidelines in 2021 expanded the criteria for FCM supplementation to HF patients with left ventricular ejection fraction (LVEF) up to 50%. 15
Basic research suggests that ID could directly contribute to arrhythmogenicity in HFrEF. Cardiomyocyte iron content is greatly reduced in HFrEF with concomitant ID 16 because of the reduction in circulating iron as well as down‐regulation of Transferrin 1 receptors (Tfr1), 17 which are required for cardiac cells to internalize iron. Given that iron is a necessary component of several enzymes that participate in oxidative phosphorylation, cardiomyocyte metabolism is directly impaired under these circumstances. 18 Our group has previously postulated that the resultant adaptations to lower energy conditions decrease cardiomyocyte's electromechanical stability, thus contributing to arrhythmogenicity. 19 IV iron treatments are theoretically ideal to restore cardiomyocyte iron status, because supra‐physiologic plasma iron concentrations enter myocardial cells through non‐Tfr1 processes. 20 Thus, a potential improvement of patients' arrhythmic burden could be regarded as a beneficial effect from IV FCM treatment in HFrEF.
Although the evidence in favour of IV FCM treatment in HFrEF is concrete, its effect on arrhythmic burden has not been explored. Data on real‐world results of IV FCM are also limited, whereas its effect on novel and established ultrasonographic parameters is also uncertain. The Iron Intravenous Therapy in Reducing the burden of Severe Arrhythmias in HFrEF (RESAFE‐HF) registry trial was designed to provide insights on the effect of IV FCM in a real‐world cohort of patients, with a particular emphasis on patients' burden of ventricular arrhythmias and myocardial function, as assessed by novel echocardiographic parameters. The study's recruitment phase has been completed. The aim of this paper is to present the study protocol and delineate the phenotype of a real‐life cohort of patients with HFrEF and ID based on baseline characteristics.
Methods
RESAFE‐HF is a prospective, single‐centre, open‐label registry study aiming to investigate the effect of IV FCM on HFrEF patients' iron stores, arrhythmic burden, hospitalizations, functional capacity, quality of life, conventional and novel ultrasonographic parameters, and disease biomarkers. The study has been designed to conform with the Declaration of Helsinki. RESAFE‐HF received approval by the responsible institutional review board and has been registered with the ClinicalTrials.gov trial registry (NCT04974021). All patients provided written informed consent.
Inclusion/exclusion criteria
HFrEF patients with ID at functional status New York Heart Association (NYHA) II–IV with CIEDs capable to record and store arrhythmic events were eligible for inclusion in RESAFE‐HF. ID was defined according to the 2016 ESC guidelines 14 as ferritin < 100 μg/L or ferritin between 100 and 299 μg/L and transferrin saturation (TSAT) < 20%. The decision to administer IV FCM was based on clinical guidelines and clinician judgement and was made prior and separately to inclusion in the RESAFE‐HF registry. Patients with a history of myocardial infarction or acute decompensated HF in the 15 days preceding IV FCM administration were excluded from the study. Patients whose CIED telemetry data had been cleared in the preceding 3 months were also ineligible to participate in the study. Untreated thyroid disease, chronic autoimmune diseases, and cancer were also exclusion criteria. Patients who refused to participate to the registry or were unwilling to continue follow‐up for their disease at the outpatient HF clinic of our department were also excluded from the study.
Study workflow
The design of the RESAFE‐HF registry study took advantage of the fact that HFrEF patients with CIEDs and ID regularly visit our outpatient HF clinic, where iron status evaluation and CIED interrogation usually take place. The fact that most patients visit the HF clinic at 3 to 6 month intervals was projected to enable the assessment of IV FCM's effect on patients 3, 6, and 12 months after baseline (defined as the time of first IV FCM administration; also, see Figure 1 ). At baseline, a complete medical history was recorded for each patient and a physical examination was performed. Hospitalization events (cause, duration) from the 12 months preceding IV FCM administrations were recorded. At baseline and throughout study follow‐up, all clinical, functional, imaging, and laboratory tests were ordered by clinicians as per standard clinical routine, while researchers recorded results to the RESAFE‐HF registry. Patient quality of life was assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ) and EQ‐5D‐5L at every patient visit, as was functional capacity via the 6 min walk test (6MWT). An electrocardiogram (ECG) was also obtained at every patient visit. As part of standard clinical practice, laboratory tests [including N‐terminal pro‐brain natriuretic peptide (NT‐proBNP)], 2D transthoracic echocardiography (2D‐TTE), CIED interrogation, late potential testing, and 24 h Holter monitoring were performed at 6 month intervals. The baseline CIED interrogation was also used to quantify patients' arrhythmic burden in the months before IV FCM administration. Regarding the baseline assessment of arrhythmic burden, ventricular tachycardia (VT), nsVT, and therapy counts were recorded. When fewer than 12 months were recorded, all available data were used for extrapolation. Assessment of exercise capacity via cardiopulmonary exercise testing (CPET) was performed at baseline and at 12 months.
Figure 1.
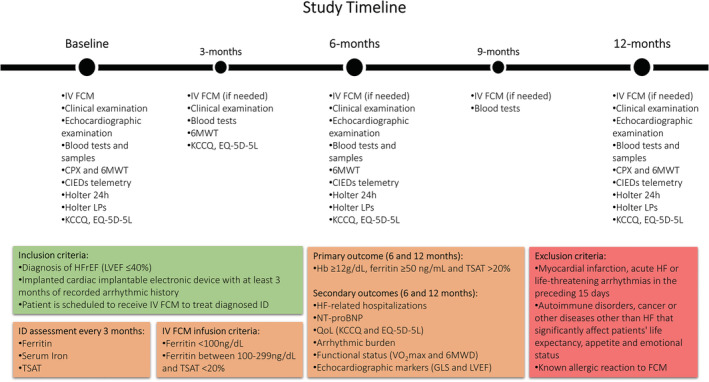
The RESAFE‐HF study timeline. 6MWD, 6 min walk distance; 6MWT, 6 min walk test; CIEDs, cardiac implantable electronic devices; CPX, cardiopulmonary exercise; FCM, ferric carboxymaltose; GLS, global longitudinal strain; Hb, haemoglobin; HFrEF, heart failure with reduced ejection fraction; ID, iron deficiency; IV, intravenous; KCCQ, Kansas City Cardiomyopathy Questionnaire; LPs, late potentials; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; QoL, quality of life; TSAT, transferrin saturation.
CIED interrogation was performed with manufacturer‐specific computer programmers by cardiac electrophysiologists, usually in the presence of cardiac rhythm management technicians. A telemetry wand was placed over the patient's device, enabling data retrieval. During each interrogation, the fullest available record stored in the device was extracted, containing information including but not limited to record duration, the number of stored episodes (with electrograms if available), trend graphs, and pacing counters. After anonymization and blinding, RESAFE‐HF researchers were able to quantify the number of nsVT, VT, and ventricular fibrillation (VF) episodes recorded for each patient, the time spent in atrial fibrillation (AF) (only applicable for patients not in permanent AF/VVI pacing), and the number of therapies [shocks and anti‐tachycardia pacing (ATP)] administered for the time period for which data were available. Blinded to patient information, a cardiac electrophysiologist reviewed the electrograms to confirm that therapy was administered in response to a rhythm requiring treatment. Inappropriate shocks and therapies were discarded.
Holter monitoring was recorded with the GE CM4000 Multi‐channel ECG recorder and analysed with the CardioDay analysis software (GETEMED Medizin‐ und Informationstechnik AG, Teltow, Brandenburg, Germany). The Holter analysis enabled the characterization of patients' arrhythmic burden via the Lown and Wolf classification. 21 This grading system ranges from 0 [no premature ventricular contractions (PVCs)] to 5 (PVCs with R‐on‐T phenomena) and reflects the severity of premature ventricular beats (PVCs) based on their frequency and complexity. Multiform and serial PVCs confer a higher (i.e. worse) score.
2D‐TTE examinations were performed on Vivid S6 and Vivid E9 ultrasound machines (GE Healthcare, Chicago, IL, USA), archived, and analysed independently by two attending physicians. The mean of the two observations was accepted. In case observations differed by more than 10%, a third expert was called upon. Their measurement was considered final. The experts were also allowed to exclude the patient from ultrasound analysis due to poor image quality. The parameters recorded for the RESAFE‐HF registry were left ventricular end‐diastolic volume index (LVEDVi), biplane LVEF, and left ventricular global longitudinal strain (LV GLS). LV GLS was calculated via the EchoPAC software suite v110.1.3 (GE Healthcare).
CPET was performed with breath‐by‐breath measurements of respiratory gas exchange using the Ultima CPX metabolic stress testing system (MGC Diagnostics, Saint Paul, MN, USA). Patients underwent bicycle ergometry with a ramping protocol tailored for each patient according to algorithms by Pretto et al. 22 The test results were accepted only if the patient stated dyspnoea or peripheral muscle fatigue as the reason for discontinuing their effort and the respiratory exchange ratio (RER) reached or surpassed 1.0 during testing. Peak VO2 and end‐tidal CO2 partial pressure (PetCO2) were recorded, whereas the VE/VCO2 slope was extrapolated by fitting a regression line using a least squares approach over all exercise data points up to cessation of effort.
Study endpoints
The composite endpoint of haemoglobin (Hb) > 12 g/dL, ferritin > 100 ng/L, and TSAT > 20% is the primary endpoint of the RESAFE‐HF registry study. Secondary endpoints constitute patients' arrhythmic burden, as appraised by (i) CIED telemetry (nsVT, VTs recorded by CIED, and appropriate device therapies during the follow‐up period compared with previous available left‐censored at 12 months) and (ii) Holter monitoring (Lown and Wolf and total PVCs). The hospitalization rate during the follow‐up period, LVEF and LV GLS, quality of life as measured with KCCQ and EQ‐5D‐5L, functional capacity as assessed with 6MWT and peak VO2, and NT‐proBNP make up the rest of secondary endpoints.
Power analysis
Power analysis would preferably be conducted with the arrhythmic burden as a basis. Unfortunately, there is limited literature around arrhythmias in HFrEF and ID. Thus, power analysis was conducted for anticipated increases in peak VO2, KCCQ, and LVEF. All power analyses were made with the Mkpower R package and an alpha of 0.05 and 80% power. Using baseline data from van Veldhuisen et al., 13 a sample size of 92 was calculated as sufficient to detect a single‐point VO2 increase, which has been found to decrease all‐cause mortality by 12–15% in HFrEF. 23 In order to reliably detect a 15% improvement in the KCCQ total score, a sample size of 94 was deemed necessary, using baseline KCCQ data from Anker et al. 11 Finally, a 5‐point LVEF increase (which has been shown to reduce the risk of hospitalization and mortality) 24 was calculated to require 98 patients for reliable detection at the pre‐specified alpha and power level. This step of power analysis utilized unpublished LVEF data from our outpatient clinic's clinical HFrEF registry, which provided a reliable estimate of the baseline LVEF (n = 156, 30 ± 12%). Taken together, the research team set a conservative target of 110 patients.
Statistical analysis
Categorical data of patients recruited to the RESAFE‐HF registry study are presented as absolute frequency (percentage of total). Continuous parameters are reported as mean ± standard deviation if normally distributed and as median [interquartile range (IQR)] if not. Non‐normally distributed parameters with a median of 0 (e.g. VF episodes per patient per year) are represented as 5% or 10% trimmed mean ± trimmed SD (percentage refers to total trimmed data points). Normality was investigated with the Shapiro–Wilk test.
Results
Patient recruitment concluded after 106 patients were included in the RESAFE‐HF registry study (Figure 2 ). A detailed report of baseline patient characteristics can be found in Table 1 . Patients' median age was 72 (IQR: 14.4) years, whereas 90 patients (84.9%) were male. ICDs were the most common implanted CIEDs (53, 50%), followed by cardiac resynchronization therapy devices with defibrillator (CRT‐D; 41–38.7%), CRT pacemakers (CRT‐P; 6–5.7%), and conventional pacemakers (6, 5.7%). Except for 30 patients with permanent AF (VVI pacing), all other patients were fitted with devices capable of and programmed to DDD pacing. Chronic kidney disease (CKD) was frequent among patients (92, 86.8%)—31 (29.2%) patients had a baseline glomerular filtration rate (GFR) below 30 mL/min. However, no patients with end‐stage renal disease were recruited in the study.
Figure 2.

Patient flowchart. CIEDs, cardiac implantable electronic devices; FCM, ferric carboxymaltose; HFrEF, heart failure with reduced ejection fraction; IV, intravenous.
Table 1.
Baseline characteristics
| Variable | All patients (N = 106) |
|---|---|
| Age (years) | 72 (14.4) |
| Sex (male, %) | 90 (84.9%) |
| BMI (kg/m2) | 29.3 (5.2) |
| NYHA | |
| I | 0 (0%) |
| II | 50 (47.2%) |
| III | 28 (45.3%) |
| IV | 8 (7.5%) |
| Ischaemic (%) | 55 (51.9%) |
| CIED | |
| Pacemaker | 6 (5.7%) |
| ICD | 53 (50%) |
| CRT‐P | 6 (5.7%) |
| CRT‐D | 41 (38.7%) |
| Diabetes | 44 (41.5%) |
| Dyslipidaemia | 74 (69.8%) |
| eGFR (mL/min/1.73 m2) | 59.4 (32) |
| AF | 74 (69.8%) |
| Paroxysmal | 44 (41.5%) |
| Permanent | 30 (39.3%) |
| Hospitalization in prec. 12 months | 38 (35.8%) |
| Medications | |
| ARNi | 52 (49.1%) |
| ACEi or ARB | 29 (27.3%) |
| ARNi or ACEi or ARB | 81 (76.4%) |
| Beta‐blockers | 105 (99.1%) |
| Aldosterone antagonists | 85 (80.2%) |
| SGLT2i | 7 (6.6%) |
| Amiodarone | 49 (46.2%) |
| NOAC | 44 (41.5%) |
ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blockers; ARNi, angiotensin receptor‐neprilysin inhibitor; BMI, body mass index; CIED, cardiac implantable electronic device; CRT‐D, cardiac resynchronization therapy defibrillator; CRT‐P, cardiac resynchronization therapy pacemaker; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter–defibrillator; NOAC, novel oral anticoagulant drugs; NYHA, New York Heart Association functional class; prec., preceding; SGLT2i, sodium‐glucose cotransporter 2 inhibitor.
Consistent with the study inclusion criteria, no patient was classified at NYHA functional class I at baseline. AF (74, 69.8%), dyslipidaemia (74, 69.8%), and type II diabetes (44, 41.5%) were common comorbidities. Regarding functional capacity, the majority of patients were NYHA II class (50, 47.2%), whereas 48 (45.3%) were classified as NYHA III and 8 (7.5%) as NYHA IV. Orthopaedic impairments prevented 9 patients (8.5%) from undergoing 6MWT and CPET, whereas 2 additional patients failed to reach an RER of 1, resulting in the invalidation of their CPET results (thus a total of 11 patients—10.4% have no CPET data).
All patients initially fulfilled the ESC criteria for IV FCM treatment, whereas 22 patients (20.8%) were within the composite iron outcome's ferritin, TSAT, and Hb targets. The mean LVEF was 28.8 ± 7.6%. In the 12 months prior to iron administration, 38 patients (35.8%) had been hospitalized for acute or decompensated HF, whereas a 10% trimmed mean of 0.58 ± 3.05 VT/VF events per patient per year was observed, leading to a 5% trimmed mean of 0.44 ± 1.43 appropriate therapies per patient per year. Significant arrhythmogenicity was apparent in the majority of patients, with 80 patients (75.5%) classified to Lown and Wolf stages above 4a, reflecting the presence of frequent, repetitive, and/or multiform PVCs. A detailed report of the baseline results of the RESAFE‐HF study endpoints can be found in Table 2 . Data from the 12 month follow‐up of all study participants are not yet available.
Table 2.
Baseline data for RESAFE‐HF endpoints
| Variable |
All patients N = 106 |
|---|---|
| Iron‐related primary outcome | |
|
Iron RESAFE composite (%) Fer ≥ 100 mL, Hb ≥ 12 g/dL, TSAT ≥ 20% |
1 (0.9%) |
|
ESC iron composite (%) Fer ≥ 300 ng/mL or Fer ≥ 100 ng/mL and TSAT ≥ 20% |
0 (0%) |
| Haemoglobin (g/dL) | 12.8 ± 1.53 |
| TSAT (%) | 18.8 (10.3) |
| Ferritin (ng/mL) | 55.4 (64) |
| CIED‐derived arrhythmic outcomes | |
|
VT/VF episodes (year−1) (10% trimmed mean ± SD) |
0.58 ± 3.05 |
|
nsVT episodes (year−1) (10% trimmed mean ± SD) |
5.22 ± 100 |
|
Appropriate shocks (year−1) (5% trimmed mean ± SD) |
0.17 ± 0.58 |
|
Appropriate therapies (year−1) (5% trimmed mean ± SD) |
0.44 ± 1.43 |
|
Days in AF last year (AF only) |
65 (365) |
| Holter‐derived arrhythmic outcomes | |
| Lown and Wolf classification | |
| 0 | 0 (0%) |
| 1 | 18 (17%) |
| 2 | 5 (4.7%) |
| 3 | 3 (2.8%) |
| 4a | 30 (28.3%) |
| 4b | 29 (27.4%) |
| 5 | 21 (19.8%) |
| Ultrasonographic outcomes | |
| LVEDVi (mm3/m2) | 92.5 (39) |
| LVEF (%) | 28.8 ± 7.6 |
| LV GLS (%) | −7.9 (3.9) |
| Functional outcomes | |
| Peak VO2 (mL/kg/min) (n = 95) | 11.1 ± 3.1 |
| VE/VCO2 slope (n = 95) | 37 (11) |
| PetCO2 (mmHg) (n = 95) | 32 (10) |
| 6MWD (m) (n = 97) | 421.3 (126) |
| Biomarker outcomes | |
| BNP (pg/mL) | 354.5 (506) |
| NT‐proBNP (pg/mL) | 777 (2424) |
| QoL outcomes | |
| KCCQ total score (%) | 65.8 (29) |
| EQ‐5D‐5L VAS (%) | 60 (20) |
6MWD, 6 min walk distance; AF, atrial fibrillation; BNP, brain natriuretic peptide; CIED, cardiac implantable electronic device; EQ‐5D‐5L VAS, EQ‐5D‐5L visual association scale; ESC, European Society of Cardiology; Fer, ferritin; Hb, haemoglobin; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV GLS, left ventricular global longitudinal strain; LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PetCO2, partial pressure of end‐tidal carbon dioxide; QoL, quality of life; SD, standard deviation; TSAT, transferrin saturation; VE/VCO2 slope, minute ventilation/carbon dioxide production; VF, ventricular fibrillation; VO2, oxygen consumption; VT, ventricular tachycardia.
Discussion
In this work, we present the rationale, design, and baseline patient characteristics of the RESAFE‐HF study. The study is expected to provide valuable, real‐word data about the effect of IV FCM on the iron reserves, functional capacity, quality of life, and echocardiographic and biochemical markers of disease severity in patients with HFrEF and ID.
Previous studies 11 , 12 , 13 have established the beneficial impact of FCM on patients with HF and ID, leading to the suggestion of its use by the ESC guidelines since 2016. 14 These studies mainly focused on the effect of FCM on patients' functional status, quality of life, hospitalizations, and deaths. 11 , 13 The IRON‐CRT trial investigated the alterations in the ejection fraction of HF patients with ID already receiving cardiac resynchronization therapy. 25 An LVEF lower than 45% was a prerequisite for inclusion in the study. In total, 37 patients received FCM and 38 received standard of care. Patients who received FCM showed a statistically significant improvement in their systolic heart function, as their LVEF improved by a median of 4.22% (P < 0.001), whereas the LVEF of the placebo group slightly decreased. 25 The IRON‐CRT trial's results stand for the clinical improvement regarding the contractility of the failing HF cardiac muscle after ID treatment with FCM. Moreover, the decompensating effect of ID on HF cardiomyocytes electromechanical status has been thoroughly investigated and discussed. 19 The RESAFE‐HF trial is designed to further clinically evaluate the effect treating ID with FCM has on the arrhythmic burden of patients with HFrEF and CIEDs, while confirming via real‐world data the benefits as for heart contractility, functional status, quality of life, HF hospitalizations, and survival. Extended analysis of specific patient categories, for example, ischaemic vs. non‐ischaemic, may reveal HFrEF phenotypes that may gain additional benefit from IV FCM administration.
The baseline patient characteristics are compatible with a population with symptomatic HFrEF and similar to the characteristics of patients who participated in the ESC Heart Failure Long‐Term Registry. 26 Some differences were noted but can be fully attributed to deviations in the registries' recruitment strategy and exclusion criteria. Consistent with the phrasing of the ESC HF guidelines regarding the use of IV FCM in HFrEF, patients with overt HF symptoms and reduced functional capacity are overrepresented in our sample (52.8% NYHA III/IV compared with 30.6% in the ESC registry). Thus, although not purely a sample of advanced HF patients, the RESAFE‐HF study population has significant symptoms. Our sample also exhibits higher age (72 vs. 64 years), which is associated with higher prevalence of comorbidities such as AF (69.8% vs. 18.3%) and type II diabetes mellitus (41.5% vs. 32.3%). The high prevalence of AF once again underlines the susceptibility of this population to arrhythmias. Comparisons with the pooled FCM sample from the meta‐analysis of randomized clinical trials 27 investigating the efficacy of IV FCM in HFrEF and ID can also be made. Overall, the RESAFE‐HF registry sample is very similar to the pooled population of the major clinical trials conducted on IV FCM. Once again, the RESAFE‐HF sample appears to be older with more comorbidities. The higher baseline 6 min walk distance (6MWD) (421.3 m vs. 277 m) is possibly owed to the higher proportion of NYHA II patients in our sample.
Several design decisions render RESAFE‐HF uniquely suited to explore the effect of IV FCM on the arrhythmic burden of patients with HFrEF and ID. Importantly, only patients with implanted CIEDs were included in the study, which is expected to aid in the investigation of the effect of IV FCM in the arrhythmic burden of HFrEF patients in two ways. Firstly, most CIEDs today hold a record of all VTs and nsVTs that fulfil sensor criteria. 28 Thus, a reliable estimation of patients' arrhythmic burden can be derived by interrogating these devices. Secondly, due to the relatively high cost of implantation, ICDs are only covered by the Greek public health system if patients have had a documented episode of aborted cardiac death, VT or VF, or inducible VT/VF during an electrophysiology study study, and thus, our patient cohort suffers increased risk of ventricular arrhythmias. It is thus opportune that our study population overwhelmingly consists of patients with HFrEF and ICDs because this is a population that stand to gain greatly from an improved arrhythmic profile.
Regarding medication use, most of our patients were under optimal medical treatment at baseline [99.1% of patients on beta‐blockers, 76.4% on angiotensin‐converting enzyme inhibitor (ACEi)/angiotensin receptor blockers (ARB)/angiotensin receptor‐neprilysin inhibitor (ARNi) of which 64.2% on ARNi, and 80.2% on aldosterone or eplerenone]. This fact is likely to reduce the likelihood of confounding treatment effects. Another noteworthy observation is the use of amiodarone by 46.2% of patients at baseline, which highlights the significant arrhythmic burden of the RESAFE‐HF study population prior to study participation.
Our study has some limitations. Importantly, this is a non‐randomized study aiming to collect real‐world data from IV FCM usage in a clinical setting. Thus, it may be unwise to uncritically establish a cause‐and‐effect relationship between IV FCM and its purported effects using the observations of RESAFE‐HF alone. The lack of a control group is unavoidable because of the fact that RESAFE‐HF is a registry study; this design consideration may further limit the ability to establish a definitive relationship between IV FCM and a potential amelioration of study participants' arrhythmic burden. However, RESAFE‐HF may still serve as a proof‐of‐concept trial with first evidence of a link between the restoration of iron stores and reduction of arrhythmogenicity in this group of patients. Additionally, we must admit that CIEDs provide valuable but imperfect records of ventricular and atrial arrhythmias. Ventricular arrhythmias below the duration and frequency criteria of a device's monitor zone may not be recorded. Monitor zone criteria can be adjusted on a patient‐by‐patient basis and potentially introduce bias. For devices lacking cardioverter/defibrillator function, the criteria for recording ventricular high‐rate events are usually fixed and vendor specific. Far‐field oversensing is a frequent cause of inappropriate mode switching and can lead to overestimation of atrial arrhythmias. 29 Although some amount of random error may be introduced in the study due to the particularities of CIED recordkeeping, systematic bias is unlikely, as the participation of patients in the RESAFE‐HF registry by no means affects their particular CIED settings. Additionally, the use of the Lown and Wolf classification as a means of assessing arrhythmic burden using data derived from Holter monitoring is not ideal, as this classification has been found to not appropriately reflect the actual risk of lethal arrhythmias in cardiovascular disease cohorts. 30 However, in the absence of superior markers, our research team opted to use it as a supplementary endpoint to the actual incidence of VT/VFs recorded in patients' CIEDs. Finally, direct assessment of the effect of IV FCM on intramyocardial iron content, as assessed through cardiac magnetic resonance (CMR) techniques, could have been an invaluable additional endpoint to this study, 31 as it would provide direct insights into the mechanism through which IV FCM improves patient outcomes. Theoretically, restitution of myocardial iron content leading to better metabolic status and thusly improved function and electrochemical stability would be directly observable with this method. 19 Unfortunately, patients' clinical status, device‐associated technical limitations, availability, and image quality constraints as well as ethical concerns about conducting multiple CMR scans on patients with implanted pacemakers, ICDs, and CRT devices led to a benefit/risk assessment that was unfavourable to the addition of CMR examinations in this group of patients.
In conclusion, the RESAFE‐HF trial was designed to investigate the effect of IV FCM on patients with HFrEF and ID under real clinical conditions. The final results of the RESAFE‐HF trial are expected to provide significant insight on the association between ID, IV FCM, and this group of patients' arrhythmic burden, as well as real data on the course of their functional status and exercise capacity post‐treatment.
Conflict of interest
This is an investigator‐initiated study, designed and conducted independently as a non‐commercial clinical study. V.P.V. reports receiving research grants from Vifor Pharma Management Ltd., in part or in whole covering publication costs, lab materials, and medical equipment conducive to the study. C.B., D.M., and A.T. have no conflicts of interest to declare. E.L. is an employee of Vifor Pharma Management Ltd.
Acknowledgements
The authors would like to thank the head nurses of the Third Cardiology Department of Aristotle University of Thessaloniki Mrs. Smaragda Giannakidou and Mrs. Efthimia Strati, as well as the nursing staff of the department in general. We also wish to acknowledge the assistance of the laboratory staff of the Second Propaedeutics Department of Internal Medicine, specifically Mrs. Aikaterini Balaska and Mr. Konstantinos Vogiatzis, with the biomarkers assessment.
Bakogiannis, C. , Mouselimis, D. , Tsarouchas, A. , Papadopoulos, C. E. , Theofillogiannakos, E. K. , Lechat, E. , Antoniadis, A. P. , Pagourelias, E. D. , Kelemanis, I. , Tzikas, S. , Fragakis, N. , Efthimiadis, G. K. , Karamitsos, T. D. , Doumas, M. , and Vassilikos, V. P. (2023) Iron therapy and severe arrhythmias in HFrEF: rationale, study design, and baseline results of the RESAFE‐HF trial. ESC Heart Failure, 10: 1184–1192. 10.1002/ehf2.14276.
Dimitrios Mouselimis and Anastasios Tsarouchas contributed equally.
References
- 1. van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MAJ, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. 2016; 18: 242–252. [DOI] [PubMed] [Google Scholar]
- 2. Lesyuk W, Kriza C, Kolominsky‐Rabas P. Cost‐of‐illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord. 2018; 18: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moradi M, Daneshi F, Behzadmehr R, Rafiemanesh H, Bouya S, Raeisi M. Quality of life of chronic heart failure patients: a systematic review and meta‐analysis. Heart Fail Rev. 2020; 25: 993–1006. [DOI] [PubMed] [Google Scholar]
- 4. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020; 22: 1342–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chieng D, Paul V, Denman R. Current device therapies for sudden cardiac death prevention—the ICD, subcutaneous ICD and wearable ICD. Heart Lung Circ. 2019; 28: 65–75. [DOI] [PubMed] [Google Scholar]
- 6. Packer M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur Heart J. 2020; 41: 1757–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ebner N, Jankowska EA, Ponikowski P, Lainscak M, Elsner S, Sliziuk V, Steinbeck L, Kube J, Bekfani T, Scherbakov N, Valentova M, Sandek A, Doehner W, Springer J, Anker SD, von Haehling S. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the Studies Investigating Co‐morbidities Aggravating Heart Failure. Int J Cardiol. 2016; 205: 6–12. [DOI] [PubMed] [Google Scholar]
- 8. Cohen‐Solal A, Philip J‐L, Picard F, Delarche N, Taldir G, Gzara H, Korichi A, Trochu J‐N, Cacoub P. Iron deficiency in heart failure patients: the French CARENFER prospective study. ESC Hear Fail. 2022; 9: 874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJV, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010; 31: 1872–1880. [DOI] [PubMed] [Google Scholar]
- 10. Okonko DO, Grzeslo A, Witkowski T, Mandal AKJ, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole‐Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency. J Am Coll Cardiol. 2008; 51: 103–112. [DOI] [PubMed] [Google Scholar]
- 11. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan B‐A, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole‐Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015; 36: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A, Voors AA, Macdougall IC, Anker SD, Roubert B, Zakin L, Cohen‐Solal A. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017; 136: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016; 37: 2129–2200.27206819 [Google Scholar]
- 15. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 16. Melenovsky V, Petrak J, Mracek T, Benes J, Borlaug BA, Nuskova H, Pluhacek T, Spatenka J, Kovalcikova J, Drahota Z, Kautzner J, Pirk J, Houstek J. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. Eur J Heart Fail. 2017; 19: 522–530. [DOI] [PubMed] [Google Scholar]
- 17. Maeder MT, Khammy O, dos Remedios C, Kaye DM. Myocardial and systemic iron depletion in heart failure. J Am Coll Cardiol. 2011; 58: 474–480. [DOI] [PubMed] [Google Scholar]
- 18. Sovari AA. Cellular and molecular mechanisms of arrhythmia by oxidative stress. Cardiol Res Pract. 2016; 2016: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bakogiannis C, Briasoulis A, Mouselimis D, Tsarouchas A, Papageorgiou N, Papadopoulos C, Fragakis N, Vassilikos V. Iron deficiency as therapeutic target in heart failure: a translational approach. Heart Fail Rev. 2019; 25: 173–182. [DOI] [PubMed] [Google Scholar]
- 20. Xu W, Barrientos T, Mao L, Rockman HA, Sauve AA, Andrews NC. Lethal cardiomyopathy in mice lacking transferrin receptor in the heart. Cell Rep. 2015; 13: 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bastiaenen R, Batchvarov V, Gallagher MM. Ventricular automaticity as a predictor of sudden death in ischaemic heart disease. Europace. 2012; 14: 795–803. [DOI] [PubMed] [Google Scholar]
- 22. Pretto JJ, Braun GW, Guy PA. Using baseline respiratory function data to optimize cycle exercise test duration. Respirology. 2001; 6: 287–291. [DOI] [PubMed] [Google Scholar]
- 23. Swank AM, Horton J, Fleg JL, Fonarow GC, Keteyian S, Goldberg L, Wolfel G, Handberg EM, Bensimhon D, Illiou M‐C, Vest M, Ewald G, Blackburn G, Leifer E, Cooper L, Kraus WE. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients. Circ Heart Fail. 2012; 5: 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Breathett K, Allen LA, Udelson J, Davis G, Bristow M. Changes in left ventricular ejection fraction predict survival and hospitalization in heart failure with reduced ejection fraction. Circ Heart Fail. 2016; 9: e002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Packer M, Butler J, Filippatos G, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Anker SD, Zannad F, the EMPEROR‐Reduced Trial Committees and Investigators , Executive Committee , Packer M, Anker SD, Butler J, Filippatos G, Zannad F, George J, Brueckmann M, National Coordinators , Perrone S, Nicholls S, Janssens S, Bocchi E, Giannetti N, Verma S, Jian Z, Spinar J, Seronde MF, Böhm M, Merkely B, Chopra V, Senni M, Taddei S, Tsutsui H, Choi DJ, Chuquiure E, la Rocca HPB, Ponikowski P, Juanatey JRG, Squire I, Butler J, Januzzi J, Pina I, Consulting Statistician , Pocock SJ, Clinical Events Committee , Carson P, Doehner W, Miller A, Haas M, Pehrson S, Komajda M, Anand I, Teerlink J, Rabinstein A, Steiner T, Kamel H, Tsivgoulis G, Lewis J, Freston J, Kaplowitz N, Mann J, Petrie M, Scientific Excellence Committee , Bernstein R, Cheung A, Green J, Januzzi J, Kaul S, Ping CLS, Lip G, Marx N, McCullough P, Mehta C, Ponikowski P, Rosenstock J, Sattar N, Scirica B, Tsutsui H, Verma S, Wanner C, Data Monitoring Committee , Welty FK, Parhofer KG, Clayton T, Pedersen TR, Lees KR, Konstam MA, Greenberg B, Palmer M. Evaluation of the effect of sodium–glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR‐Reduced trial. Eur J Heart Fail. 2019; 21: 1270–1278. [DOI] [PubMed] [Google Scholar]
- 26. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: an analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail. 2017; 19: 1574–1585. [DOI] [PubMed] [Google Scholar]
- 27. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Lüscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail. 2018; 20: 125–133. [DOI] [PubMed] [Google Scholar]
- 28. Zeitler EP, Piccini JP. Remote monitoring of cardiac implantable electronic devices (CIED). Trends Cardiovasc Med. 2016; 26: 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kolb C, Wille B, Maurer D, Schuchert A, Weber R, Schibgilla V, Klein N, Hümmer A, Schmitt C, Zrenner B. Management of far‐field R wave sensing for the avoidance of inappropriate mode switch in dual chamber pacemakers: results of the FFS‐test study. J Cardiovasc Electrophysiol. 2006; 17: 992–997. [DOI] [PubMed] [Google Scholar]
- 30. Dabrowski A, Kołodziej P, Krupienicz A, Drzewiecki J, Giec L, Trusz‐Gluza M, Wnuk‐Wojnar A, Kopeć P, Sredniawa B. Comparative evaluation of the results of Holter monitoring and programmed ventricular stimulation in patients with ischemic heart disease. Kardiol Pol. 1990; 33: 34–39. [PubMed] [Google Scholar]
- 31. Pereira GAR, Foppa M, Eifer DA, Beck‐da‐Silva L. Myocardial iron content by T2 Star cardiac magnetic resonance and serum markers of iron metabolism in patients with heart failure. J Cardiovasc Med (Hagerstown). 2022; 23: e33–e35. [DOI] [PubMed] [Google Scholar]


