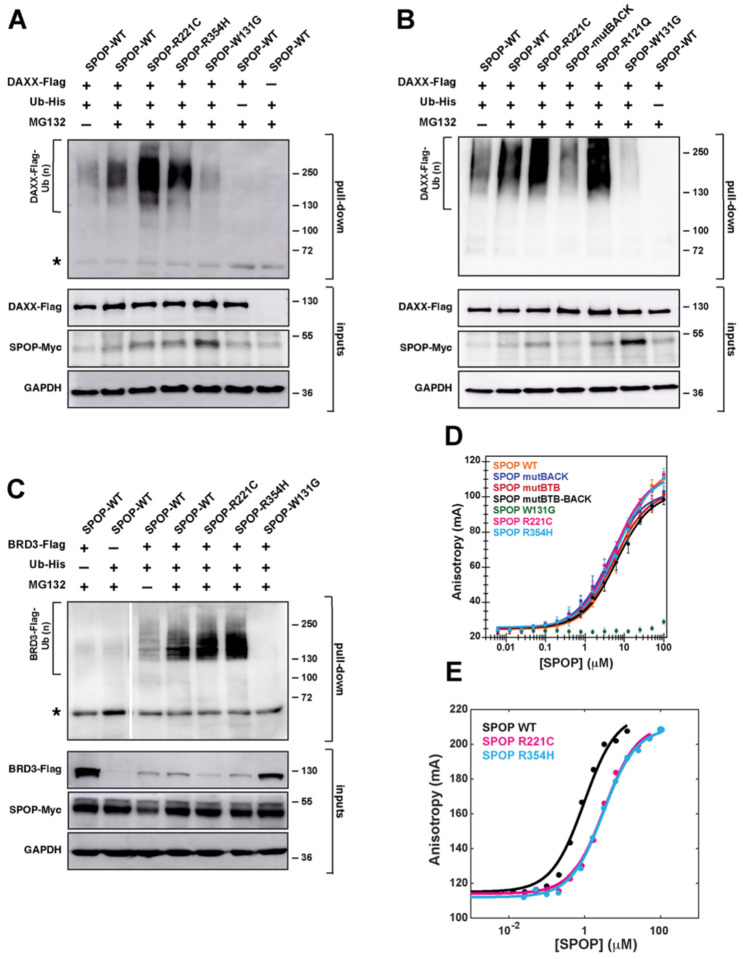
Figure 4. SPOP interface mutations enhance ubiquitination but reduce substrate binding.
(A) SPOPR221C and SPOPR354H enhance DAXX polyubiquitination. Representative immunoblot showing ubiquitination in T-REx cells transfected to express SPOP-Myc, DAXX-Flag, and His-tagged ubiquitin. Twenty-four hours post-transfection, cells were incubated with DMSO or 20 μM MG132 for 4–5 hours. The cells were lysed and the resulting lysates were used in His-tag pull-down assays, using nickel-NTA beads under denaturing conditions. The samples were then run on SDS–PAGE gels, and the gels were immunoblotted with an anti-Flag antibody. Protein input was verified using antibodies for Myc, Flag, and GAPDH (loading control). (B) Similar to the interface mutant R221C, the endometrial cancer mutant R121Q promotes SPOP polyubiquitination activity. The opposite effect is observed for mutBACK, the SPOP mutant that completely disrupts BACK domain dimerization. Experimental conditions as in (A). (C) SPOPR221C and SPOPR354H enhance BRD3 polyubiquitination, indicating that gain-of-function for the interface mutants is not limited to the substrate DAXX. Experimental conditions as in (A), except that BRD3-Flag is expressed as the substrate instead of DAXX-Flag. Asterisks in panels A and C indicate a cross-reacting band. (D) SPOP affinity for its canonical binding motif is not altered for the SPOPR221C and SPOPR354H mutants relative to SPOPWT, but it is reduced in SPOPW131G, a prostate cancer mutant that abrogates substrate binding. The affinities were measured using a fluorescence anisotropy binding assay with a fluorescently labeled peptide that was derived from a SPOP-binding motif from the substrate Puckered. (E) The binding affinity for SPOPR221C and SPOPR354H to the multivalent substrate is slightly decreased. Affinities were measured using a fluorescence anisotropy binding assay with fluorescently labeled cDAXX.