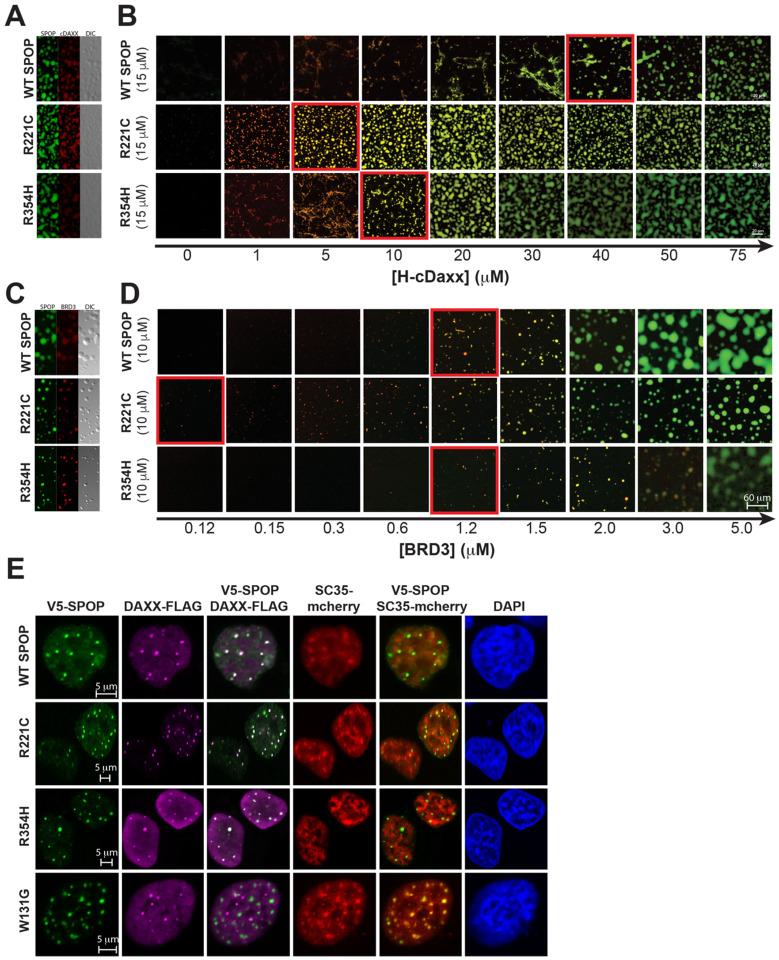
Figure 5. SPOP interface mutants enhance phase separation with substrates.
(A) Two-channel confocal fluorescence and DIC microscopy images show that SPOPWT, SPOPR221C, and SPOPR354H each colocalize with cDAXX in SPOP/cDAXX condensates. (B) The phase boundary between SPOP/cDAXX clusters and condensates is shifted for the SPOP proteins with interface mutations. Confocal fluorescence microscopy images of SPOPWT or an interface mutant (green) as a function of cDAXX (red) concentration. The boundary between clusters and condensates is indicated by a red outline. All samples contain 10% w/v ficoll 70, 500 nM ORG-SPOP, and/or 500 nM Rhodamine-cDAXX. (C) Two-channel confocal fluorescence and DIC microscopy images show that SPOPWT, SPOPR221C, and SPOPR354H each colocalize with BRD3 in SPOP/BRD3 condensates. (D) The phase boundary between SPOP/BRD3 clusters and condensates is shifted for the SPOP proteins with interface mutations. Confocal fluorescence microscopy images of SPOPWT or an interface mutant (green) as a function of BRD3 (red) concentration. The boundary between clusters and condensates is indicated by a red outline. All samples contain 200 nM ORG-SPOP and/or 100 nM Rhodamine-BRD3. (E) Representative fluorescence confocal images of HeLa cells expressing V5-SPOP, DAXX-Flag, and SC35-mCherry constructs (the latter to mark nuclear speckles). Cells were transfected with the indicated plasmids. Twenty-four hours post-transfection, cells were fixed and immuno-stained using antibodies against V5 (green) and Flag (magenta). DAPI (blue) marks nuclear DNA.