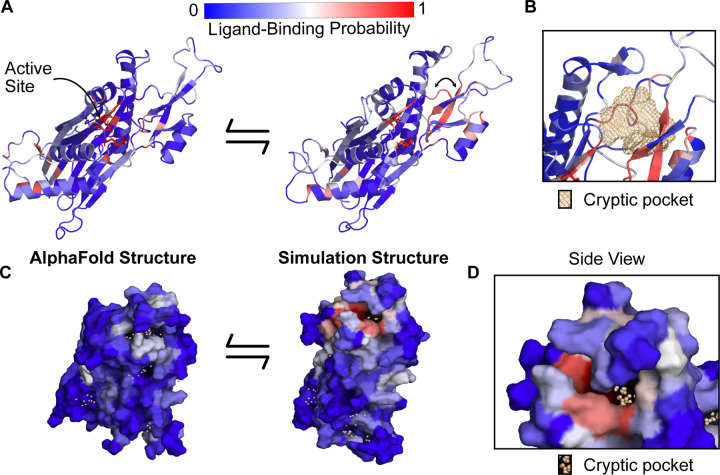
Figure 3: PPM1D apo simulations reveal a cryptic pocket at the flap-hinge interface.
A) The AlphaFold-predicted PPM1D structure and a simulation structure where each residue is colored by its P2Rank-predicted ligand-binding probabilities show an increase in ligand-binding probability at the flap domain near the hinge. This simulation structure was selected because it had the largest increases in ligand-binding probability relative to the starting structure across the ensemble of states. Active site residues are shown in sticks. Arrow indicates the backbone motion that is required to form the cryptic pocket. B) Mesh representation of the cryptic pocket shows that it forms between a flap domain loop (residues 276–279), two of the β-strands in the flap (residues 243–247 and 268–271), and a flap domain helix (residues 227–234). C) Surface representation looking onto the AlphaFold structure and the open simulation structure highlights that a deep trench forms between the flap domain and hinge.The surface is colored by P2Rank-predicted ligand-binding probability. D) A zoom-in of the surface representation of the open state reveals that the cryptic pocket lies in a deep groove. The orange spheres are the pocket grid points identified by P2Rank.