Extended Data Figure 4. Effects of CRISPRi-mediated knockdown of Malat1 on proosteoclastogenic signaling and Nfatc1.
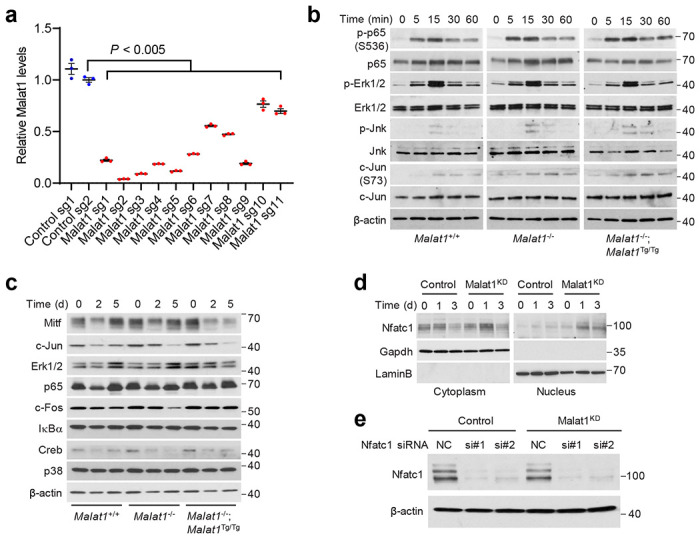
a. qPCR of Malat1 in B16F1 cells with CRISPRi-mediated knockdown of Malat1. Statistical significance was determined by a two-tailed unpaired t-test. Error bars are s.e.m.
b. Malat1+/+, Malat1−/−, and Malat1−/−;Malat1Tg/Tg BMMs were cultured with M-CSF (50 ng/mL) before stimulation with RANKL (100 ng/mL) for 0, 5, 15, 30, and 60 min. The cell lysates were subjected to immunoblotting with antibodies against phospho-p65, p65, phospho-Erk1/2, Erk1/2, phospho-Jnk, Jnk, phospho-c-Jun, c-Jun, and β-actin.
c. Malat1+/+, Malat1−/−, and Malat1−/−;Malat1Tg/Tg BMMs were treated with M-CSF (50 ng/mL) and RANKL (100 ng/mL) for 2 days and 5 days. The cell lysates were subjected to immunoblotting with antibodies against Mitf, c-Jun, Erk1/2, p65, c-Fos, IκBα, Creb, p38, and β-actin.
d. Control and Malat1-knockdown RAW264.7 cells were cultured with RANKL (50 ng/mL) for 1 day and 3 days. After fractionation, the cytoplasmic and nuclear fractions were subjected to immunoblotting with antibodies against Nfatc1, Gapdh (a cytoplasmic marker), and Lamin B (a nuclear marker).
e. Immunoblotting of Nfatc1 and β-actin in control and Malat1-knockdown RAW264.7 cells transfected with Nfatc1 siRNA or scrambled negative control (NC).
