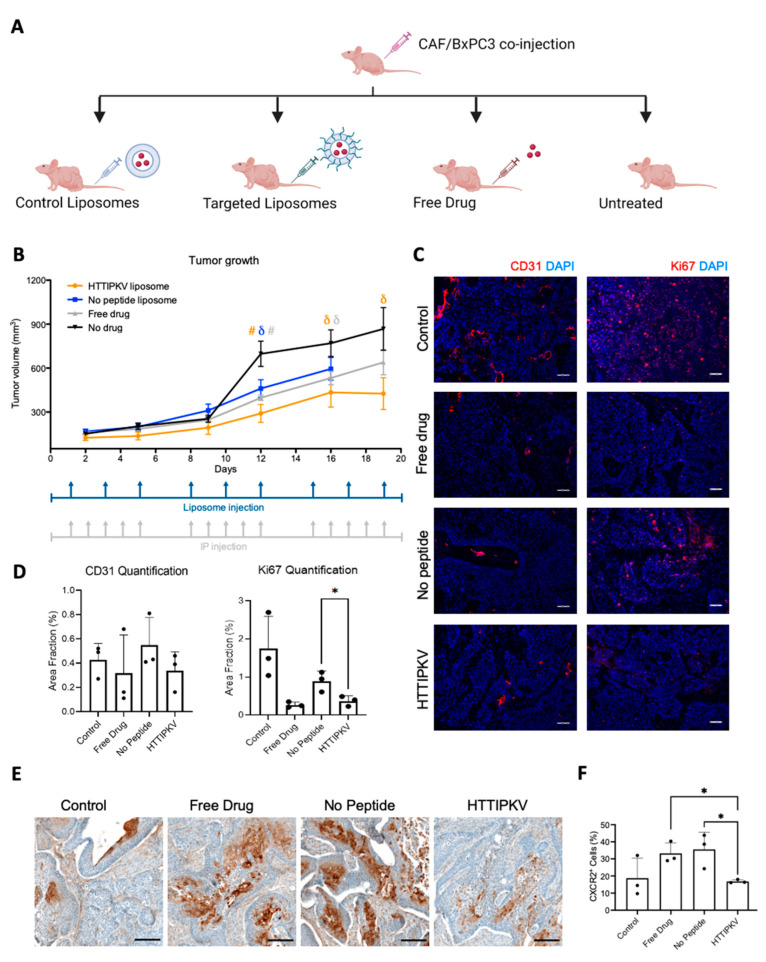
Figure 5.
CXCR2 inhibition in an admix BXPC3/CAF xenograft model following SB225002 delivery. (A) Schematic of mouse study design. (B) Mice bearing subcutaneous admix BXPC3/CAF tumors were injected with SB225002, control liposomes loaded with SB225002, targeted liposomes loaded with SB225002, or no treatment. Tumor growth was measured via calipers for 19 days (n = 10–12 tumors/group). Dosage schedules for liposomes and free drug (IP injections) are as indicated. # p < 0.01, and δ p < 0.05 statistical significance between liposomes with or without a peptide compared to no drug by Student’s t-test. The untargeted liposome group was terminated early due to tumor ulceration. (C) Representative images of CD31 and Ki67 staining show the vessels and cell proliferation in different treatment groups. Scale bar, 50 µm. (D) Quantification of images using thresholding in ImageJ was averaged across five images of three sections of three tumors (45 images total per treatment group). * p < 0.05 statistical significance with Student’s t-test. (E) Representative images of anti-CXCR2 IHC staining. Scale bar, 150 µm. (F) Quantification of the percent of positively stained cells relative to the total number of cells per tissue, regardless of localization using QuPath v.0.2.3. (n = 3 tumors/group) [44]. * p < 0.05 statistical significance with Student’s t-test.