Abstract
Background Although a growing number of very elderly patients with atrial fibrillation (AF), multiple conditions, and polypharmacy receive direct oral anticoagulants (DOACs), few studies specifically investigated both apixaban/rivaroxaban pharmacokinetics and pharmacodynamics in such patients.
Aims To investigate: (1) DOAC concentration–time profiles; (2) thrombin generation (TG); and (3) clinical outcomes 6 months after inclusion in very elderly AF in-patients receiving rivaroxaban or apixaban.
Methods Adage -NCT02464488 was an academic prospective exploratory multicenter study, enrolling AF in-patients aged ≥80 years, receiving DOAC for at least 4 days. Each patient had one to five blood samples at different time points over 20 days. DOAC concentrations were determined using chromogenic assays. TG was investigated using ST-Genesia (STG-ThromboScreen, STG-DrugScreen).
Results We included 215 patients (women 71.1%, mean age: 87 ± 4 years), 104 rivaroxaban and 111 apixaban, and 79.5% receiving reduced-dose regimen. We observed important inter-individual variabilities (coefficient of variation) whatever the regimen, at C max [49–46%] and C min [75–61%] in 15 mg rivaroxaban and 2.5 mg apixaban patients, respectively. The dose regimen was associated with C max and C min plasma concentrations in apixaban ( p = 0.0058 and p = 0.0222, respectively), but not in rivaroxaban samples (multivariate analysis). Moreover, substantial variability of thrombin peak height (STG-ThromboScreen) was noticed at a given plasma concentration for both xabans, suggesting an impact of the underlying coagulation status on TG in elderly in-patients. After 6-month follow-up, major bleeding/thromboembolic event/death rates were 6.7%/1.0%/17.3% in rivaroxaban and 5.4%/3.6%/18.9% in apixaban patients, respectively.
Conclusion Our study provides original data in very elderly patients receiving DOAC in a real-life setting, showing great inter-individual variability in plasma concentrations and TG parameters. Further research is needed to understand the potential clinical impact of these findings.
Keywords: apixaban, atrial fibrillation, elderly, rivaroxaban, thrombin generation
Introduction
Nonvalvular atrial fibrillation (NVAF) is the most common heart rhythm disorder with a prevalence exceeding 10% in octogenarians. 1 Current guidelines recommend the use of direct oral anticoagulants (DOACs) targeting either factor Xa (FXa; such as rivaroxaban or apixaban) or thrombin (dabigatran) as first-line treatment for the prevention of stroke and systemic thromboembolism in patients with NVAF. 2 3 DOACs are characterized by predictable pharmacokinetics (PK) and pharmacodynamics (PD) with a wide therapeutic index: therefore, they are administered at fixed doses without laboratory monitoring. 4 5 6 7 8 9 10 11 However, the management of very elderly patients receiving anticoagulant treatment may be challenging because they are at both high risk of thrombotic and bleeding complications even though meta-analysis and real-life studies provided reassuring data regarding the clinical benefit–risk balance of DOACs. 12 13 14 15 Phase II/III clinical trials and phase IV studies evaluating the PK/PD profiles of DOACs in healthy volunteers and NVAF patients have provided consistent PK and PD data with substantial inter-individual variability. 16 17 18 19 20 21 22 23 24 25 26 However, little is known regarding specific concentration–time profiles of rivaroxaban and apixaban in polymedicated NVAF patients aged ≥80 years. 27 28 29 This age group is characterized by the presence of multiple comorbidities (including renal impairment) and polypharmacy, potentially altering both drug exposure and effect. Both xabans are metabolized via cytochrome P450 (CYP) 3A4/5 and CYP2J2, and substrates of the drug-efflux transporter P-glycoprotein (P-gp). Several studies have evaluated the effect of moderate CYP3A4/P-gp inhibitor concomitant use on rivaroxaban or apixaban exposure, especially in healthy volunteers. 4 20 One question is whether drug–drug interactions could influence PK response variability in the very elderly.
Regarding the assessment of DOAC PD, the study of thrombin generation (TG) has been shown as a suitable and reliable assay to assess in vitro or ex vivo anticoagulant effect of direct FXa inhibitors in contrast to prothrombin time. 30 31 32 33 34 35 36 Nevertheless, to the best of our knowledge, no specific TG data regarding elderly patients aged 80 years and over receiving rivaroxaban or apixaban have been published yet.
Hence, we conducted an academic prospective exploratory study in a cohort of NVAF-hospitalized patients aged ≥80 years receiving rivaroxaban or apixaban. The primary objectives were to characterize rivaroxaban and apixaban concentration–time profiles and determine the potential impact of demographic, clinical, therapeutic, and pharmacogenetic factors on their peak and trough concentrations. The secondary objectives were to perform TG assays in a subset of patients under several experimental conditions and evaluate the potential influence of individual characteristics on TG parameter variability at peak and trough levels. The tertiary objectives were to report clinical outcomes (bleeding and thromboembolic complications, death) at 6 months.
Methods
Adage Study Design and Inclusion Criteria
The Assessment of Direct oral Anticoagulants in GEriatrics (Adage) study is an academic multicenter prospective observational study ( www.clinicaltrials.gov ; NCT02464488). We recruited hospitalized patients in university hospital geriatric departments (Assistance Publique-Hôpitaux de Paris AP-HP, France; CHU-UCL Namur-site-Godinne, Belgium) (January 2015 to June 2019). Patients aged ≥80 years and receiving rivaroxaban or apixaban for NVAF since at least 4 days were eligible. The DOAC dose regimen was at the discretion of the physician. Exclusion criteria included any acute unstable comorbid condition or an estimated life expectancy of a few weeks. The study was approved by the local ethics committee (Comité consultatif de protection des personnes pour la recherche biomédicale – Île-de-France-6). All participants gave their written informed consent to participate in the study.
Physicians prospectively collected demographic data, laboratory data (including creatinine clearance [CrCl] calculated with the Cockcroft–Gault formula), comorbid conditions allowing the calculation of the CHA 2 DS 2 VASc, HEMORR 2 HAGES, and the 13-item Cumulative Illness Rating Scale-Geriatrics (CIRS-G) scores. 37 We recorded DOAC regimen and concomitant medications (excluding vitamins). None of the patients had received heparin derivatives in the last 5 days prior the first sampling. Drugs were considered as modulators (substrate or moderate/strong inhibitors) of P-gp/CYP3A4/5 according to Geneva University Hospitals classification. 38 Finally, geriatricians were responsible for the clinical follow-up of the patients at 6 months through a phone call and/or the use of medical records, and recorded bleedings (site, severity), thromboembolic events (site), and deaths. Two geriatricians (F.K., L.R.) blinded to the PK/PD study results adjudicated bleeding events as major or nonmajor clinically relevant bleedings according to the International Society on Thrombosis and Haemostasis criteria 39 ; minor bleedings were not systematically recorded.
Sample Collection in A dage Patients
Blood samples were collected by venipuncture into a citrate tube (buffered trisodium citrate 0.109 M, 9 vol/1vol) in addition to other samples taken as part of usual care between 7.00 a.m. and 7.00 p.m. No additional venipuncture was specifically performed for our study purpose. Sampling and last DOAC intake times were systematically recorded on a standardized request form. Sampling time points were at the discretion of the physicians. At most five samples per patient were collected over a maximal 20-day period following inclusion. Time points were classified according to the time elapsed from the last oral intake ( T , hours). Especially, for rivaroxaban patients, T1 to T4 samples corresponded to time to peak (TTP) level (namely T max ) and T20 to T24 corresponded to time to trough level ( T min ); for patients receiving apixaban (twice daily [b.i.d.]), T1 and T4 samples corresponded to T max , and T10 to T12 samples to T min .
Tubes were sent to each center's laboratory within 2 hours after sampling. They were immediately double-centrifuged for 15 minutes at 2,000 g at 15 to 22°C to obtain platelet-poor plasma (PPP) distributed in 500 μL aliquots; pellets were kept for genotyping. All samples were stored at −80°C, prior to onward shipment on dry ice for central analysis (Haematology laboratory, Hôpital Lariboisière AP-HP, Paris, France). PPP samples were thawed for 3 to 5 minutes in a 37°C water-bath just before use.
Rivaroxaban and Apixaban Concentrations and Thrombin Generation Assessment
Anti-Xa chromogenic assays (STA-Liquid-Anti-Xa, Stago, Asnières-sur-Seine, France) were used to determine rivaroxaban and apixaban plasma concentrations using dedicated calibrators and controls on STA-R-Evolution (Stago) (lower limit of quantification: 20 ng/mL). The maximal observed plasma DOAC concentration ( C max ) was defined as the level measured at T max (T1–T4), whereas the minimal one ( C min ) was defined as the level measured at T min (i.e., T20–T24 for rivaroxaban, T10–T12 for apixaban).
TG was studied using the ST-Genesia system (Stago) whenever sufficient plasma was available. ST-Genesia is a fully automated system enabling quantitative standardized assessment of TG derived from Hemker's fluorescence method, using dedicated reagents, calibrator, and quality controls. 35 Three experimental conditions (as a function of the reagent used) were tested: STG-DrugScreen and STG-ThromboScreen, the latter in presence/absence of thrombomodulin (+TM/− TM) according to the manufacturer's instructions. STG-DrugScreen contains a mixture of phospholipids and recombinant human tissue factor (TF) at a high pM concentration; STG-ThromboScreen contains TF at a lower, intermediate, pM concentration. 35 TM is contained in the reagent at a concentration chosen to inhibit 50% of the endogenous thrombin potential (ETP) assessed in normal pooled plasma. Four parameters were analyzed: lag time (LT; in minutes, time from test triggering to signal detection), TTP (in minutes, time necessary for thrombin concentration to reach its maximal value), peak height (PH; in nM: maximal thrombin concentration), and ETP (in nM·min: area under the thrombin time–concentration curve). 35 Results are presented as absolute values and inhibition percentages in presence of TM.
TG in Plasma Samples Spiked with Xabans
Frozen normal pooled plasma samples (Cryocheck, Montpellier, France) were spiked with increasing concentrations of rivaroxaban or apixaban ranging from 0 to 450 ng/mL as previously described. 35 We measured effective plasma concentrations in each sample with specific anti-Xa assays.
ABCB1 , CYP3A5 , and CYP2J2 Genotyping
DNA was extracted from peripheral blood leukocytes using the kits E.Z.N.A. SQ (Omega Bio-tek, Norcross, Georgia, United States). We analyzed four loss-of-function variants in three genes potentially involved in the DOAC metabolism ( CYP2J2, CYP3A5 ) or transport ( ABCB1 encoding P-gp) variability: CYP2J2 -76G > T (rs890293;*7), CYP3A5 6981A > G (rs776746;*3), ABCB1 rs2032582 (2677G > T/A), and ABCB1 rs1045642 (3435C > T) (PharmGKB; www.pharmvar.org ). They were analyzed using real-time polymerase chain reaction with TaqMan Assay-Reagents for allelic discrimination (Applied Biosystems, Courtabœuf, France).
Statistical Analysis
All statistical analyses were run on R-software (version 3.5.1) using the nlme package. 40 41 Quantitative data were described as median (interquartile range [IQR]) or mean values (±standard deviation), and minimal and maximal values. Genetic variants were coded as 0 (wild type), 1 (heterozygous), or 2 (homozygous) to model additive allelic effects. Frequencies of genotypes were compared to the Hardy–Weinberg equilibrium expected frequencies using the χ 2 test. The association between concentrations (at T max and T min ) or PD parameters and each covariate was tested using either Spearman's rank correlation (quantitative covariate) or the Kruskal–Wallis test (qualitative covariate). We prespecified that PH measured using STG-ThromboScreen without TM would be chosen as the PD parameter for statistical analyses (given its optimal sensitivity to a wide range of DOAC concentrations according to previous data of our group). 35 Multivariate analysis was done using multiple linear regression, after log-transformation of the predicted parameter (concentrations or PH). Predictors included in this analysis were selected based on either their association with the studied parameter ( p- value less than 0.10 in the univariate analysis) or their clinical pertinence. Results are given as 95% confidence interval of the parameters in the model. Model quality and assumptions were graphically checked. A p -value less than 0.05 was considered statistically significant. All p -values are given uncorrected.
Results
Patient Characteristics
From November 2015 to July 2019, 215 in-patients (153 females, 62 males) with NVAF were included in the Adage study, with a mean age of 86.6 ± 4.3 years (min–max: 80–100). Patient characteristics are summarized in Table 1 . The rivaroxaban group comprised 104 patients: 18 (17.3%) received the 20-mg full dose o.d., 83 (79.8%) the 15-mg dose, and 3 (2.9%) the 10-mg dose. The apixaban group comprised 111 patients: 26 (23.4%) received the 5-mg full dose b.i.d. and 85 (76.6%) the 2.5-mg dose b.i.d.; 72 (33.5%) patients did not receive the dose recommended according to the Summary of Product Characteristics 10 11 (i.e., 15-mg rivaroxaban in patients with CrCl ≤50 mL/min, and 2.5-mg apixaban in patients ≥80 years with creatinine level ≥133 µM or body weight ≤60 kg): 62 patients received a lower dose regimen than recommended (28 on rivaroxaban and 34 on apixaban), whereas 10 patients received a higher one (8 on rivaroxaban and 2 on apixaban).
Table 1. Characteristics of Adage patients at inclusion .
| Total | Rivaroxaban | Apixaban | p -Value | |
|---|---|---|---|---|
| n = 215 | n = 104 | n = 111 | ||
| Demographic characteristics | ||||
| Age (y) | 86.6 ± 4.3 | 86.4 ± 4.4 | 86.8 ± 4.3 | 0.491 |
| Females (%) | 71.2 | 68.3 | 73.9 | 0.371 |
| Body weight (kg) | 65.6 ± 14.7 | 64.9 ± 12.6 | 66.4 ± 16.5 | 0.543 |
| DOAC regimen | ||||
| Full dose (%) a | 20.5 | 17.3 | 23.4 | 0.312 |
| Reduced dose (%) b | 79.5 | 82.7 | 76.6 | 0.312 |
| Clinical characteristics | ||||
| Type of AF | ||||
| Persistent (%) | 9.1 | 8.7 | 9.4 | 0.978 |
| Paroxysmal (%) | 34.0 | 35.0 | 33.0 | 0.978 |
| Permanent (%) | 56.9 | 56.3 | 57.6 | 0.978 |
| Previous stroke or TIA (%) | 24.9 | 21.4 | 28.2 | 0.250 |
| Diabetes mellitus (%) †† | 19.0 | 10.9 | 26.6 | 0.005 |
| Hypertension (%) | 76.1 | 72.8 | 79.1 | 0.336 |
| Heart failure (%) | 49.8 | 46.6 | 52.7 | 0.412 |
| Dyslipidemia (%) | 26.4 | 24.3 | 28.4 | 0.535 |
| Clinical scores | ||||
| CHA 2 DS 2 VASc | 5.0 ± 1.4 | 4.9 ± 1.4 | 5.2 ± 1.4 | 0.122 |
| HEMORR2HAGES † | 2.3 ± 1.0 | 2.1 ± 1.0 | 2.5 ± 1.0 | 0.022 |
| CIRS-G † | 10.4 ± 4.5 | 9.3 ± 3.7 | 11.4 ± 4.8 | 0.007 |
| Laboratory data | ||||
| Creatinine clearance (mL/min) c | 49.1 ± 16.5 | 50.5 ± 17.1 | 47.9 ± 15.9 | 0.314 |
| Albumin (g/L) | 33.2 ± 5.2 | 33.4 ± 4.2 | 33.0 ± 5.9 | 0.756 |
| C-reactive protein (mg/L) | 28.8 ± 39.8 | 28.6 ± 41.9 | 28.9 ± 38.0 | 0.374 |
| Hemoglobin (g/dL) | 12.1 ± 1.7 | 12.2 ± 1.7 | 12.0 ± 1.8 | 0.400 |
| Fibrinogen (g/L) | 5.0 ± 1.5 | 4.9 ± 1.6 | 5.0 ± 1.4 | 0.309 |
| Therapeutic data | ||||
| Number of co-medications | 6.2 ± 2.7 | 5.9 ± 2.5 | 6.5 ± 2.9 | 0.256 |
| ≥1 P-gp substrate/inhibitor including amio. (%) | 48.3 | 44.6 | 51.8 | 0.292 |
| ≥1 CYP3A4/5 inhibitor including amio. (%) | 22.3 | 17.8 | 26.4 | 0.136 |
| Amiodarone (%) | 21.8 | 17.8 | 25.5 | 0.187 |
| Antiplatelet drugs (%) | 15.0 | 10.0 | 20.0 | 0.055 |
Abbreviations: AF, atrial fibrillation; Amio, amiodarone; TIA, transient ischemic attack.
Note: Data shown as percentage or mean ± standard deviation. Statistically significantly different values between the rivaroxaban and apixaban groups are indicated: † p < 0.05 or †† p < 0.01.
20 mg o.d. (rivaroxaban) or 5 mg b.i.d. (apixaban).
15 or 10 mg o.d. (rivaroxaban) or 2.5 mg b.i.d. (apixaban).
Estimated using the Cockcroft–Gault formula.
Most patients had multiple comorbidities (mean CIRS-G score: 10.4 ± 4.5); 68.8% had moderate renal impairment (CrCl: 30–59 mL/min). Most patients were polymedicated (mean number of comedications: 6.2 ± 2.7 per patient). Overall, patient characteristics did not significantly differ between the two DOAC groups, except for CIRS-G and HEMORR 2 HAGE scores which were higher in the apixaban group ( p = 0.0023 and p = 0.0218, respectively). ABCB1 2677G > T/A , ABCB1 3435C > T , CYP2J2*7 , and CYP3A5*3 allelic frequencies in Adage patients are shown in Supplementary Table S1 (available in the online version).
Concentration–Time Profiles of Rivaroxaban and Apixaban in Adage Patients
A total of 456 blood samples were collected (average 2.1 samples per patient): 232 from 104 rivaroxaban patients and 224 from 111 apixaban patients. Fig. 1 shows DOAC plasma concentration–time profiles. Among Adage patients receiving 15-mg rivaroxaban o.d., median C max was 251 ng/mL (IQR: 176–377; range: 64–676); median C min was 42 ng/mL (IQR: 28–70, range: <20–241). Among patients receiving 2.5-mg apixaban b.i.d., the median C max was 180 ng/mL (IQR: 130–227; range: 69–469) and the median C min was 74 ng/mL (IQR: 49–116; range: <20–251). At T max , 30.6% of patients on rivaroxaban 15 mg o.d. and 31.8% of patients on 2.5-mg apixaban b.i.d. had plasma concentrations above the 95th percentile of values reported in the Summary of Product Characteristics obtained in younger patients included in pivotal clinical trials. 10 11 21 At T min , only 3.1 and 9.7% were above the 95th percentile, whereas 21.5 and 9.7% were below the 5th percentile, respectively.
Fig. 1.
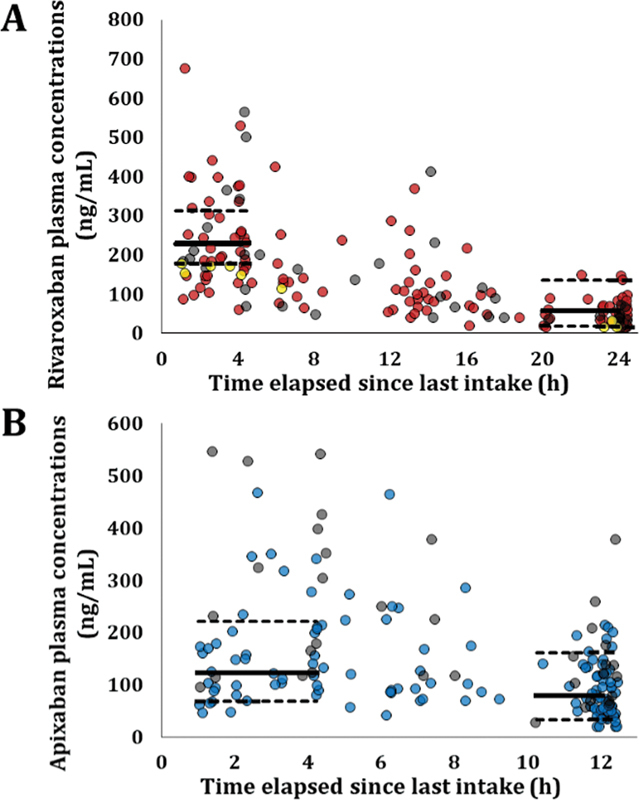
Plasma concentrations of rivaroxaban (A) and apixaban (B) in Adage patients as a function of time since the last oral intake. ( A ) Patients receiving rivaroxaban 10, 15, or 20 mg o.d. are represented with yellow, red, and grey circles, respectively (noteworthy, 10 mg off-labeled in AF). In order to facilitate interpretation, we represented the geometric mean C max and C min and the 5th–95th percentiles, i.e ., 229 ng/mL (5th–95th: 178–313) and 57 ng/mL (5th–95th: 18–136), as reported in ROCKET-AF patients (median age: 73 years) receiving 15 mg according to Girgis et al 21 with solid and dashed lines, respectively. ( B ) Patients receiving apixaban 2.5 or 5 mg b.i.d. are represented with blue and grey circles, respectively. We represented the median C max and C min and 5th–95th percentiles, i.e. , 123 ng/mL (5th–95th: 69–221) and 79 ng/mL (5th–95th: 34–162), as reported in patients receiving apixaban 2.5 mg b.i.d. in ARISTOTLE trial 11 (median age: 70 years), with solid and dashed lines, respectively. AF, atrial fibrillation; b.i.d., twice daily; o.d., once daily.
Association of Covariates with Rivaroxaban Plasma Concentration at C max and C min
In the rivaroxaban group, we observed an important inter-individual variability of C max (coefficients of variation [CVs] 49 and 47%) and C min (75 and 37%) for 15- and 20-mg regimens, respectively. No covariates were found significantly ( p < 0.05) associated with C max in univariate analysis ( Table 2 ).
Table 2. Influence of Adage patients' characteristics on xaban plasma concentrations at T max and T min .
| Covariates | Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C max | C min | C max | C min | |||||||
| Effect | % (explained variability) |
p | Effect | % (explained variability) |
p | Effect | p | Effect | p | |
| Rivaroxaban | ||||||||||
| Dose regimen |
+20.3%
a
[−16.7; +73.5] |
2.23% | 0.346 |
−3.8%
a
[−35.1; +42.6] |
0.72% | 0.974 |
29.4%
a
[−14.2; 95.2] |
0.210 |
−3.2%
a
[−35.1; 44.6] |
0.873 |
| Female gender | +12.2% [−17.7; 53.0] |
4.09% | 0.28 | −14.2% [−36.0; 15.1] |
3.34% | 0.424 | +11.8% [−20.8; 57.9] |
0.515 | −10.1% [−35.2; 22.3] |
0.466 |
| Creatinine clearance |
+1.89%
b
[−8.2; 13.1] |
0.72% | 0.83 |
+8.1%
b
[−3.9; 15.8] |
0.57% | 0.093 |
+3.4%
b
[−7.6; 15.6] |
0.553 |
+5.0%
b
[−4.9; 16.0] |
0.325 |
| Amiodarone | +4.73% [−31.5; 60.2] |
0.07% | 0.99 |
+47.8%
[−2.8; 109.4] |
2.23% | 0.0315 |
−26.1%
b
[−75.8; 125.5] |
0.345 |
99.8%
c
[10.3; 262.0] |
0.023 |
| P-gp inhibitor c | −9.95% [−35.3; 25.4] |
1.42% | 0.60 | −15.9% [−37.6; 13.5] |
1.47% | 0.264 | −6.31% [−35.8; 36.6] |
0.200 | −8.7% [−34.2; 26.8] |
0.162 |
| CYP3A4/5 modulator c | +9.5% [−21.0; 51.6] |
1.20% | 0.52 |
+31.1%
[−24.7; 72.2] |
3.45% | 0.0361 | +10.6% [−23.1; 58.9] |
0.417 | +43.1% [5.3; 94.3] |
0.023 |
| ABCB1 3435C > T |
+18.3%
d
[−5.3; 36.6] |
4.25% | 0.20 |
+16.9%
d
[−6.6; 31.0] |
1.39% | 0.0788 |
+18.3%
d
[−10.9; 39.8] |
0.188 |
+10.4%
d
[−14.9; 30.1] |
0.381 |
| Apixaban | ||||||||||
| Dose regimen |
+44.9%
e
[7.14; 96.1] |
12.20% | 0.025 |
+45.6%
e
[4.7; 102.6] |
7.45% | 0.0317 |
+64.8%
e
[16.6; 132.9] |
0.006 |
+54.8%
e
[6.65; 124.6] |
0.022 |
| Female gender |
+44.0%
[7.4; 93.1] |
9.80% | 0.017 | +15.7% [−14.9; 57.1] |
1.12% | 0.348 | +28.7% [−4.5; 73.4] |
0.095 | 14.5% [−17.3; 58.6] |
0.410 |
| Creatinine clearance |
+3.3%
b
[−4.28; 10.31] |
0.98% | 0.289 |
+4.6%
b
[−4.2; 12.7] |
3.23% | 0.166 |
+4.5%
b
[−3.0; 11.4] |
0.223 |
+4.5%
b
[−4.9; 13.1] |
0.332 |
| Amiodarone |
+43.5%
[7.9; 90.9] |
10.25% | 0.0098 | +14.4% [−18.5; 60.5] |
0.58% | 0.433 | +79.3% [5.5; 204.7] |
0.032 | +44.1% [−31.3; 202.2] |
0.492 |
| P-gp inhibitor c | −12.7% [−37.1; 21.0] |
2.12% | 0.408 | +5.7% [−26.6; 52.1] |
0.23% | 0.764 | −4.0% [−36.3; 44.8] |
0.187 | −10.7% [−43.7; 41.6] |
0.808 |
| CYP3A4/5 modulator c | +4.0% [−20.4; 35.9] |
0.23% | 0.769 | +20.8% [−9.8; 61.7] |
2.42% | 0.203 | +12.1% [−17.3; 51.9] |
0.284 | +23.4% [−11.1; 71.3] |
0.330 |
| ABCB1 3435C > T |
+2.1%
d
[−18.7; 19.3] |
0.13% | 0.938 |
+14.8%
d
[−4.1; 30.3] |
1.70% | 0.087 |
+10.1%
d
[−8.3; 25.3] |
0.256 |
+15.2%
d
[−4.1; 30.9] |
0.113 |
Note: significance threshold p < 0.05 (bold values)
15 mg o.d. vs. 20 mg o.d.
Per decrease of 10 mL/min.
Patients who received a P-gp or CYP3A4/5 modulator in addition to amiodarone.
Per mutated allele. The 3 patients who received 10 mg o.d. were excluded from the statistical analysis.
2.5 mg b.i.d. vs. 5 mg b.i.d.
Rivaroxaban concentrations slightly increased with the daily dose (+20.3% in 20-mg patients compared to 15 mg, p = 0.346). At T min , patients on amiodarone displayed significantly higher rivaroxaban levels compared to the other patients (60 vs. 41 ng/mL; +47.8%; p = 0.0315). The intake of a CYP3A4/5 modulator other than amiodarone also contributed to increase C min ( p = 0.0361). C min increased when renal function worsened (+8.1% by 10 mL/min CrCl decrease, p = 0.093). Finally, there was a trend to higher concentrations in ABCB13435C > T carriers, without reaching significance (+16.9% per mutated allele, p = 0.0788). The multivariate analysis included gender, amiodarone intake, CYP3A4/5- or P-gp-modulator intake in addition to amiodarone, DOAC daily dose (20 vs. 15 mg), CrCl, and ABCB1 3435C > T genotype. The model also included interaction terms between amiodarone and other modulators intake. This analysis showed significantly higher C min in patients receiving either amiodarone ( p = 0.0232) or a CYP3A4/5 modulator other than amiodarone ( p = 0.0228).
Association of Covariates with Apixaban Plasma Concentrations at C max and C min
We observed a substantial inter-individual variability in Adage patients on apixaban with CVs of 46 and 47% at T max and 61 and 68% at T min for 2.5 and 5.0 mg b.i.d. regimens, respectively. In univariate analysis, the apixaban dose regimen was significantly associated with apixaban concentrations, which were 44.9% higher ( p = 0.0247) at T max and 45.6% higher at T min ( p = 0.0317) in patients receiving the full dose ( Table 2 ). Two other variables were associated with higher apixaban C max : amiodarone therapy (mean C max 251 vs. 175 ng/mL, +43.5%, p = 0.0098) and female gender (mean C max 208 ng/mL vs. 145, +44.0% in women, p = 0.0165). At T min , higher concentrations were observed in ABCB1 3435C > T carriers, without reaching statistical significance (+14.8% per mutated allele, p = 0.0867). No other variables apparently explained the apixaban concentration variability at T max or T min . The multivariate analysis included the same predictors than for rivaroxaban. The dose regimen was significantly associated with apixaban concentrations at both T max (+64.8%, p = 0.0058) and T min (+54.8%, p = 0.0222). Amiodarone might also contribute to higher concentrations at T max ( p = 0.0319).
Thrombin Generation Profiles of Rivaroxaban and Apixaban in Adage Patients
TG parameters were measured in 250 plasma samples from 128 Adage patients: 128 samples from 62 rivaroxaban patients and 122 from 66 apixaban patients. Patient characteristics did not differ significantly between both groups (not shown).
Temporal parameters (LT and TTP) were prolonged and PHs were decreased in a DOAC concentration-dependent manner ( p < 10 −4 ), using either STG-DrugScreen ( Supplementary Fig. S1 , available in the online version) or intermediate pM TF concentration (STG-ThromboScreen without TM [ Fig. 2 ], LT and TTP not shown); ETP was associated with apixaban concentrations under these both experimental conditions ( p = 0.0102 and p < 10 −4 , respectively), but not with rivaroxaban concentrations ( p = 0.1028 and 0.1846, respectively).
Fig. 2.
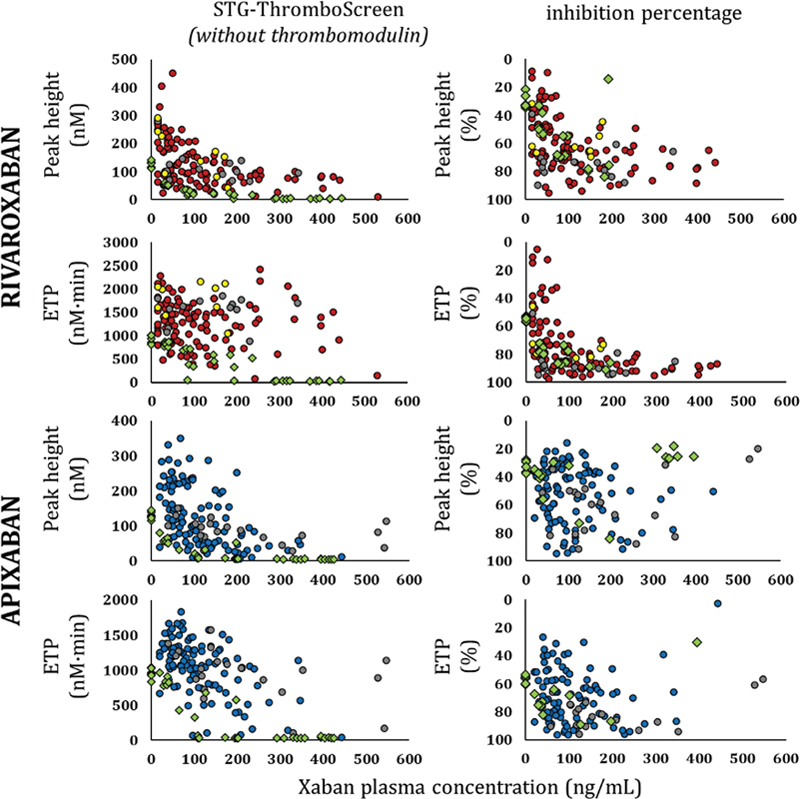
Peak height and endogenous thrombin potential as a function of DOAC concentrations in Adage patients and in normal pooled plasma samples spiked with DOAC, using STG-Thrombo-Screen reagent in the absence of thrombomodulin, and inhibition percentage in the presence of thrombomodulin. Absolute values of TG parameters in the absence of thrombomodulin (TM) (left) and percentage of inhibition in the presence of TM (right). Samples from patients receiving 10, 15, or 20 mg of rivaroxaban o.d. are represented with yellow, red, and grey circles, respectively. Samples from patients receiving 2.5 or 5 mg of apixaban b.i.d. are represented with blue and grey circles, respectively. Samples from pooled normal plasma spiked with DOAC at varying concentrations are represented with green diamonds. 35 b.i.d., twice daily; DOAC, direct oral anticoagulant; o.d., once daily; TG, thrombin generation.
Interestingly, Adage patients' PH and ETP values presented substantial variability with both xabans for a given plasma concentration, independently of the dose regimen and the experimental conditions ( Supplementary Fig. S1 [available in the online version] and Fig. 2 ). Adding TM to TG triggered with intermediate TF concentration markedly reduced ETP and PH, with strong associations with rivaroxaban and apixaban concentrations ( p < 10 −4 ) ( Fig. 2 ). A marked variability in ETP and PH percentages of inhibition was also observed, reflecting the important variability in the protein C-based negative feedback in very elderly patients.
Association of Covariates with DOAC TG Peak Height/ETP at T max and T min
Supplementary Table S2 (available in the online version) summarizes TG results focused on patient variability at T max and at T min . PH CV confirmed the substantial variability, both at T max and T min . We sought to identify covariates, namely DOAC plasma concentrations together with individual characteristics, potentially associated with PH and ETP measured using STG-ThromboScreen in the absence of TM at T max and T min ( Table 3 ). In the univariate analysis performed in the rivaroxaban group at T max , two variables were significantly associated with PH decrease: renal failure assessed using CrCl (−16.5% per 10 mL/min decrease, p = 0.0016) and heart failure (−36.6%, p = 0.0414). In multivariate analysis, CrCl was the only variable associated with PH at T max ( p = 0.0085). At T min , amiodarone intake was associated with PH decrease (−52.2%, p = 0.0116) as well as rivaroxaban concentrations ( p = 0.0005), the latter explaining 18.1% of PH variance in univariate analysis.
Table 3. Association of Adage patients' characteristics including xaban plasma concentration and peak height (using STG-ThromboScreen reagent without thrombomodulin) .
| Covariates | Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T max | T min | T max | T min | |||||||
| Effect | % | p | Effect | % | p | Effect | p | Effect | p | |
| Rivaroxaban | ||||||||||
| Plasma concentrations |
+23.9%
a
[−11.4; 73.2] |
8.4% | 0.537 |
+38.7%
a
[12.4;71.1] |
22.1% | 0.0018 |
–5.0%
a
[−27.9; 25.2] |
0.700 |
+21.0%
a
[−4.0; 52.4] |
0.103 |
| Creatinine clearance |
−12.8%
b
[−26.3; 3.0] |
22.9% | 0.027 |
−6.1%
b
[−16.2; 5.2 ] |
3.2% | 0.184 |
−16.4%
b
[−26.3; −5.0] |
0.0085 |
−6.76%
b
[−15.5; 2.8] |
0.156 |
| Heart failure | −37.5% [−59.8; −2.7] |
19.34% | 0.041 | −31.4% [−52.2; −1.7] |
10.8% | 0.055 | −22.3% [−45.0; 9.8] |
0.143 | −12.7% [−37.7; 22.4] |
0.422 |
| Amiodarone c | N.A c | N.A c . | N.A. | −51.5% [−70.1; −21.2] |
12.7% | 0.014 | N.A. c | N.A. c | −44.6%** [−75.1; 23.2] |
0.228 |
| Apixaban | ||||||||||
| Plasma concentrations |
+80.2%
a
10.0; 195.0] |
22.6% | 0.022 |
37.1%
a
13.1; 66.2] |
20.8% | 0.0005 |
+86.8%
a
[−0.48; 250.5] |
0.052 |
+32.7%
a
[8.28; 62.59] |
0.0074 |
| Creatinine clearance |
+0.42%
b
[4.70; 36.9] |
0.08% | 0.903 |
1.7%
b
[−10.7; 15.8] |
0.49% | 0.566 |
+2.4%
b
[−17.9; 27.8] |
0.825 |
−1.9%
b
[−13.4; 11.1] |
0.753 |
| Heart failure | −26.7% [−66.7; 61.4] |
1.8% | 0.426 | 3.2% [−30.5; 53.3] |
0.01% | 0.935 | −9.2% [−62.9; 122.6] |
0.826 | +0.84% [−30.8; 46.9] |
0.965 |
| Amiodarone c | +3.7% [−58.7; 160.1] |
0.5% | 0.936 | −37.2% [−60.0; −1.5] |
6.30% | 0.043 | −26.0% [−91.8; 564.1] |
0.759 | −57.47% [−83.2; 7.9] |
0.129 |
Note: Significance threshold: p < 0.05, bold values.
For halved concentrations.
Per decrease of 10 mL/min.
N.A = not available (number of values from patients on amiodarone at T max < 5).
The univariate analysis performed in the apixaban group showed a PH decrease significantly associated with increasing concentrations of apixaban, both at T max and T min ( p = 0.0224 and p = 0.0005) ( Table 3 ), accounting for 12.4 and 16.6% of the observed variance, respectively. In addition, patients receiving amiodarone had a lower PH at T min than those without amiodarone intake (−37.2%, p = 0.0426). The multivariate analysis confirmed that apixaban concentrations were the only determinant of the PH variability at T min ( p = 0.0074) and T max ( p = 0.0516) ( Table 3 ).
Six-Month Follow-Up
We could not obtain data on bleedings and thrombosis in 11 patients (5 on rivaroxaban, 6 on apixaban). The overall rate of major bleedings was 6.0% (rivaroxaban: n = 7 [6.7%]; apixaban: n = 6 [5.4%]); the rate of clinically relevant nonmajor bleeding was 3.3% (rivaroxaban: n = 2 [1.9%]; apixaban: n = 5 [4.5%]). The main bleeding sites were gastrointestinal ( n = 7), intracranial ( n = 3), and genitourinary tract ( n = 3). Five patients (2.3%) (rivaroxaban: n = 1 [1.0%], apixaban: n = 4 [3.6%]) had a thromboembolic event. Finally, 39 patients died (18.1%), 18 on rivaroxaban (17.3%) and 21 on apixaban (18.9%), with a median 3.4 month delay (1.8–4.6) after inclusion. No relationships were found between clinical events and PK/PD parameters.
Discussion
Adage is, to the best of our knowledge, the first study specifically focused on the assessment of both rivaroxaban/apixaban concentrations and effects evaluated by TG in a real-life cohort of very elderly NVAF patients. Patients were very old (mean age, 87 years), had substantial comorbidities and polypharmacy, frequently including the intake of amiodarone and/or another CYP3A4/5 or P-gp inhibitor. Thus, we believe that the characteristics of the participants in Adage study are representative of the complex medical issues associated with anticoagulant treatment in NVAF patients of 80 years and over.
One strength of the Adage study was to provide DOAC PK data which are scarce in this specific setting of hospitalized very elderly patients. 28 29 Indeed, knowing the extent of DOAC concentrations in octo- and nonagenarians should facilitate the interpretation of DOAC concentrations when monitoring such patients in specific situations. Mean C max and C min of Adage patients receiving rivaroxaban 15 mg were remarkably close to those reported in Rocket-AF patients (mean age 73 years) receiving 20 mg, 7 whereas mean concentrations of Adage patients receiving 20 mg o.d. were higher, especially at T max . Moreover, we observed a larger inter-individual variability of C max and C min for both regimens compared to Mueck et al's data, i.e., around 30%. 7 Our data show similar or even greater CVs compared to those of previous observational studies conducted in patients rather younger than ours. 21 22 23 25 26 27 42 43
Interestingly, we found that the intake of amiodarone, which is both a CYP3A4/5 and a P-gp inhibitor, as well as the additional intake of another CYP3A4/5 modulator, significantly contributed to the increase of rivaroxaban trough levels, explaining part of the concentration variability consistently with data from pivotal trials. 20 Besides, renal function is known to influence the clearance of rivaroxaban, justifying dose adjustment in case of moderate renal impairment: we only observed a trend towards higher concentrations at T min with low CrCl, suggesting that the 25% rivaroxaban dose reduction was appropriate in our patients.
Regarding apixaban, Adage patients receiving 2.5 mg b.i.d. had a median concentration at T max close to that of patients receiving 5 mg b.i.d., while the residual concentration in Adage patients was close to that reported in the Summary of Product Characteristics for 2.5 mg b.i.d.; patients receiving 5 mg b.i.d. had higher median C max and C min . 11 In addition, inter-individual variabilities were higher than those reported in SmPC (i.e., around 30% from the ARISTOTLE data set, mean age 70 years) and in previous studies. 11 18 19 23 24 27 28 29 42 43 Interestingly, the apixaban dose regimen was the only covariate that was significantly associated with plasma C max and C min in multivariate analysis, in contrast to rivaroxaban; moreover, the apixaban regimen subsequently significantly impacted TG peak height. If the apixaban dose regimen had been given according to the patient characteristics, one would have expected similar concentrations as for rivaroxaban. One explanation is that the apixaban reduced dose (2.5 mg b.i.d.) corresponds to half of the full dose (5 mg bid), while the rivaroxaban reduced dose (15 mg) corresponds to three-quarters of the full dose (20 mg). It is important to note that a substantial proportion of patients were theoretically underdosed for both xabans (31% of patients on apixaban, 27% on rivaroxaban). The fear of hemorrhagic complications probably explains the higher proportion of off-label reduced dose among Adage patients compared with those described in real-life registries. 44 45 46 47 Herein, we showed that the impact of the DOAC regimen choice, reduced versus full dose, on plasma concentrations and PD parameters was higher with apixaban than with rivaroxaban. However, our study was underpowered to conclude whether dose reduction impacts clinical outcomes: this deserves specific future investigations in geriatrics.
Among covariates, female sex was significantly associated with high apixaban C max in univariate analysis, as already reported by Frost et al in a PK study 19 and real-life studies. 48 Moreover, we also showed that the intake of amiodarone was a significant determinant of the DOAC variability of C max in univariate analysis. However, although Adage patients were highly polymedicated with several co-prescribed CYP3A4/5 and/or P-gp inhibitors, no strong association was shown with DOAC concentrations in multivariate analysis within the limits of the study. Our results reinforce the literature data suggesting that rivaroxaban and apixaban do not interact with those drugs to the extent of a clinically relevant impact justifying a subsequent dose reduction. It should be noted that no patients were taking strong CYP3A4/5/P-gp inhibitors or inducers, highlighting the compliance with DOAC contraindications in this cohort.
One of the original aims of our work was to explore the potential influence of pharmacogenetic factors on DOAC concentrations. We showed that the presence of CYP3A5*3 and CYP2J2*7 variants was not significantly associated with the variability of rivaroxaban or apixaban, in agreement with previous studies. 48 49 50 51 We observed a trend to higher concentrations in ABCB1-3435T carriers: interestingly, this variant has been shown to be associated with a reduced risk of thromboembolic outcomes in a large Finnish cohort study. 52 Interestingly, an impact of an ABCG2 variant coding breast resistance coding protein was reported in a younger cohort and could be investigated in Adage patients. 50 53
Another strength of our study was to evaluate the TG profile of rivaroxaban and apixaban in a subset of Adage patients using a standardized TG analyzer (ST-Genesia). We confirmed that both xabans exerted a concentration-dependent effect on TG parameters using both TF conditions; PHs were consistently more affected than ETP in agreement with previous findings in various series of much younger patients. 31 35 54 55 56 57 The optimal choice between intermediate (STG-ThromboScreen) and high (STG-DrugScreen) pM-TF concentrations may depend on DOAC concentration, preferentially intermediate up to 300 ng/mL and high above 300 ng/mL. 57 Remarkably, we were able to evidence a high inter-individual variability of TG parameters among patients receiving both full and reduced-dose xabans. Spiking pooled normal plasma with increasing concentrations of xabans allowed a better understanding of the inter-individual variability and highlighted an underlying hypercoagulable state in some Adage patients, leading to shorter LT and TTP combined with higher PH and ETP. We had primarily checked that the pooled normal plasma contained normal procoagulant factors and natural coagulation inhibitor levels. 35 Moreover, for a given concentration, we observed under the same conditions higher PH and ETP results in Adage rivaroxaban patients when compared to those obtained in Driving rivaroxaban healthy volunteers, aged 18 to 45 years, thus supporting this hypothesis. 35 57 It is well known that advancing age and chronic comorbid conditions lead to an imbalance between procoagulant factors and coagulation natural inhibitors, potentially causes of hypercoagulability. 58 This issue regarding TG variability deserves further studies in geriatric setting.
The overall major bleeding rate of 6.0% at 6 months was not surprisingly higher than that reported in phase III trials in patients >75 years given the characteristics of Adage patients. 8 9 The predominance of gastrointestinal bleedings could be expected in these patients, as shown in previous studies and meta-analysis. 13 14 15 59 The mortality rate (18.1%) was similar in patients treated with rivaroxaban and apixaban. The recruitment of Adage patients in geriatrics units probably reflects a high degree of frailty in Adage patients.
Our study has several limitations. First, although one to five samples per patient were planned over a 20-day period with an average of three samples per patient, a smaller number of blood samples were drawn ( i.e., 2.1) because of short hospital stays. Moreover, the sample size clearly limits analyses of associations of outcomes with measures. Second, the results regarding the influence of individual factors on concentrations at T max and T min and PD obtained in this exploratory study must be confirmed in an external cohort of hospitalized patients. Third, most patients were recruited during hospitalization: these results cannot be extrapolated to stable outpatients of similar age even though CIRS-scores took into account both chronic and acute comorbid conditions. Finally, our study was not designed to detect possible associations between PK/PD parameters measured only over a short period at inclusion and clinical events at 6 months.
In conclusion, our study provides new real-life data regarding DOAC concentrations and TG inter-individual variability in elderly in-patients with NVAF, multiple comorbidities, and medications. The DOAC regimen choice (reduced vs. full dose) was significantly associated with plasma concentrations and TG PHs for apixaban, but not for rivaroxaban. The underlying coagulation status of elderly patients could affect TG profiles. Further research is needed to better understand the substantial PK and PD variability and its potential clinical impact in the rapidly growing population of very old patients with multiple conditions who need long-term anticoagulant treatment.
Acknowledgement
We are grateful to Guillaume Paris for technical assistance.
Funding Statement
Funding Reagents were purchased thanks to a CONNY-MAEVA Charitable Foundation grant and the Société Française de Cardiologie. The funding sources had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data.
Conflict of Interest V.S., I.G.-T., E.Pa, E.Pu, F.M., M.D., and P.G. have received honoraria for participating in expert meetings on dabigatran (from Boehringer Ingelheim), rivaroxaban (from Bayer Healthcare AG), or apixaban (Bristol Myers Squibb-Pfizer). G.F. and E.Pu received a grant from Bayer. The other authors declare no conflicts of interest.
Authors Contribution
G.F.-P., V.S., G.J., and E.C. wrote the manuscript; C.L-L, V.S., E.P., and I.G.-T designed the research; C.L-L, J.L, E.P., and M.D. were clinical investigators; G.F.-P., V.S., G.J., F.T., F.K., and L.R. performed the research; G.F.-P., V.S., G.J., T.L., and E.C. analyzed the data; all authors reviewed the manuscript.
These authors share equal authorship.
What is known about this topic?
Very elderly patients (≥80 years) are characterized by numerous comorbid conditions (e.g., renal impairment) and polypharmacy, potentially altering drug PK/PD.
Few specific DOAC pharmacokinetics (PK) and pharmacodynamics (PD) data are available in this age group with numerous comorbid conditions and polypharmacy.
What does this paper add?
Our academic prospective study provides original data in very elderly in-patients receiving xaban in real-life setting, showing great inter-individual variability in plasma concentrations and PD parameters.
Substantial variability of thrombin peak-heights was noticed at a given plasma concentration for both xabans, suggesting an impact of the underlying coagulation status of elderly in-patients.
The dose regimen (reduced dose vs. full dose) had a significant impact on plasma concentrations at T max and T min in apixaban, but not in rivaroxaban patients.
Supplementary Material
References
- 1.Chugh S S, Havmoeller R, Narayanan K. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(08):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ESC Scientific Document Group . Hindricks G, Potpara T, Dagres N. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(05):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 3.January C T, Wann L S, Calkins H. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(02):e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 4.Hanna M S, Mohan P, Knabb R, Gupta E, Frost C, Lawrence J H. Development of apixaban: a novel anticoagulant for prevention of stroke in patients with atrial fibrillation. Ann N Y Acad Sci. 2014;1329:93–106. doi: 10.1111/nyas.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubitza D, Becka M, Roth A, Mueck W. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin. 2008;24(10):2757–2765. doi: 10.1185/03007990802361499. [DOI] [PubMed] [Google Scholar]
- 6.Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78(04):412–421. doi: 10.1016/j.clpt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet. 2014;53(01):1–16. doi: 10.1007/s40262-013-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ROCKET AF Investigators . Patel M R, Mahaffey K W, Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 9.ARISTOTLE Committees and Investigators . Granger C B, Alexander J H, McMurray J JV. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 10.Bayer A G.Xarelto 20 mg film-coated tablets SmPCPublished online January 17, 2020. Accessed October 8, 2020 at:https://www.ema.europa.eu/en/documents/product-information/xarelto-epar-product-information_en.pdf
- 11.Bristol-Myers Squibb/Pfizer EEIG Eliquis 5 mg film-coated tablets SmPCPublished online September 16, 2020. Accessed October 8, 2020 at:https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf
- 12.Sharma M, Cornelius V R, Patel J P, Davies J G, Molokhia M. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: systematic review and meta-analysis. Circulation. 2015;132(03):194–204. doi: 10.1161/CIRCULATIONAHA.114.013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell A, Watson M C, Welsh T, McGrogan A. Effectiveness and safety of direct oral anticoagulants versus vitamin k antagonists for people aged 75 years and over with atrial fibrillation: a systematic review and meta-analyses of observational studies. J Clin Med. 2019;8(04):E554. doi: 10.3390/jcm8040554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apixaban for Reduction of Stroke and Other Thromboembolic Complications in Atrial Fibrillation (ARISTOTLE) Investigators . Alexander J H, Andersson U, Lopes R D. Apixaban 5 mg twice daily and clinical outcomes in patients with atrial fibrillation and advanced age, low body weight, or high creatinine: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1(06):673–681. doi: 10.1001/jamacardio.2016.1829. [DOI] [PubMed] [Google Scholar]
- 15.Deitelzweig S, Keshishian A, Li X. Comparisons between oral anticoagulants among older nonvalvular atrial fibrillation patients. J Am Geriatr Soc. 2019;67(08):1662–1671. doi: 10.1111/jgs.15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubitza D, Becka M, Roth A, Mueck W. The influence of age and gender on the pharmacokinetics and pharmacodynamics of rivaroxaban–an oral, direct Factor Xa inhibitor. J Clin Pharmacol. 2013;53(03):249–255. doi: 10.1002/jcph.5. [DOI] [PubMed] [Google Scholar]
- 17.Mueck W, Schwers S, Stampfuss J. Rivaroxaban and other novel oral anticoagulants: pharmacokinetics in healthy subjects, specific patient populations and relevance of coagulation monitoring. Thromb J. 2013;11(01):10. doi: 10.1186/1477-9560-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frost C, Wang J, Nepal S. Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75(02):476–487. doi: 10.1111/j.1365-2125.2012.04369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost C E, Song Y, Shenker A. Effects of age and sex on the single-dose pharmacokinetics and pharmacodynamics of apixaban. Clin Pharmacokinet. 2015;54(06):651–662. doi: 10.1007/s40262-014-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong I Y, Kim R B.Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban Can J Cardiol 201329(7, Suppl):S24–S33. [DOI] [PubMed] [Google Scholar]
- 21.Girgis I G, Patel M R, Peters G R. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with non-valvular atrial fibrillation: results from ROCKET AF. J Clin Pharmacol. 2014;54(08):917–927. doi: 10.1002/jcph.288. [DOI] [PubMed] [Google Scholar]
- 22.Al-Aieshy F, Malmström R E, Antovic J. Clinical evaluation of laboratory methods to monitor exposure of rivaroxaban at trough and peak in patients with atrial fibrillation. Eur J Clin Pharmacol. 2016;72(06):671–679. doi: 10.1007/s00228-016-2060-y. [DOI] [PubMed] [Google Scholar]
- 23.START-Laboratory Register . Testa S, Tripodi A, Legnani C. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: results observed in four anticoagulation clinics. Thromb Res. 2016;137:178–183. doi: 10.1016/j.thromres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Cirincione B, Kowalski K, Nielsen J. Population pharmacokinetics of apixaban in subjects with nonvalvular atrial fibrillation. CPT Pharmacometrics Syst Pharmacol. 2018;7(11):728–738. doi: 10.1002/psp4.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Testa S, Paoletti O, Legnani C. Low drug levels and thrombotic complications in high-risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16(05):842–848. doi: 10.1111/jth.14001. [DOI] [PubMed] [Google Scholar]
- 26.Miklič M, Mavri A, Vene N. Intra- and inter- individual rivaroxaban concentrations and potential bleeding risk in patients with atrial fibrillation. Eur J Clin Pharmacol. 2019;75(08):1069–1075. doi: 10.1007/s00228-019-02693-2. [DOI] [PubMed] [Google Scholar]
- 27.Bendayan M, Mardigyan V, Williamson D. Muscle mass and direct oral anticoagulant activity in older adults with atrial fibrillation. J Am Geriatr Soc. 2021;69(04):1012–1018. doi: 10.1111/jgs.16992. [DOI] [PubMed] [Google Scholar]
- 28.Nissan R, Spectre G, Hershkovitz A. Apixaban levels in octogenarian patients with non-valvular atrial fibrillation. Drugs Aging. 2019;36(02):165–177. doi: 10.1007/s40266-018-0613-8. [DOI] [PubMed] [Google Scholar]
- 29.Sukumar S, Gulilat M, Linton B. Apixaban concentrations with lower than recommended dosing in older adults with atrial fibrillation. J Am Geriatr Soc. 2019;67(09):1902–1906. doi: 10.1111/jgs.15982. [DOI] [PubMed] [Google Scholar]
- 30.Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62(05):699–707. doi: 10.1373/clinchem.2015.248625. [DOI] [PubMed] [Google Scholar]
- 31.Pfrepper C, Metze M, Siegemund A, Klöter T, Siegemund T, Petros S. Direct oral anticoagulant plasma levels and thrombin generation on ST Genesia system. Res Pract Thromb Haemost. 2020;4(04):619–627. doi: 10.1002/rth2.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binder N B, Depasse F, Mueller J. Clinical use of thrombin generation assays. J Thromb Haemost. 2021;19(12):2918–2929. doi: 10.1111/jth.15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meihandoest T, Studt J D, Mendez A. Automated thrombin generation assay for rivaroxaban, apixaban, and edoxaban measurements. Front Cardiovasc Med. 2021;8:717939. doi: 10.3389/fcvm.2021.717939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemker H C, Al Dieri R, De Smedt E, Béguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost. 2006;96(05):553–561. [PubMed] [Google Scholar]
- 35.Foulon-Pinto G, Jourdi G, Perrin J. Study of thrombin generation with St Genesia to evaluate xaban pharmacodynamics: analytical performances over 18 months. Int J Lab Hematol. 2021;43(04):821–830. doi: 10.1111/ijlh.13443. [DOI] [PubMed] [Google Scholar]
- 36.Douxfils J, Adcock D M, Bates S M. 2021 Update of the International Council for Standardization in Haematology recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2021;121(08):1008–1020. doi: 10.1055/a-1450-8178. [DOI] [PubMed] [Google Scholar]
- 37.Miller M D, Paradis C F, Houck P R. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(03):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 38.Service de pharmacologie et toxicologie cliniques - Hôpitaux universitaires de Genève. Interactions médicamenteuses, cytochromes P450 et P-glycoprotéine (Pgp). Published 2020. Accessed June 20, 2022 at:https://www.hug.ch/sites/interhug/files/structures/pharmacologie_et_toxicologie_cliniques/images/carte_des_cytochromes_2020.pdf
- 39.Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(04):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 40.Pinheiro J, Bates D, DebRoy S, Sarkar D.Nlme: linear and nonlinear mixed effects models. 2021. Accessed December 13, 2022 at:https://CRAN.R-project.org/package=nlme
- 41.R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Accessed December 13, 2022 at:https://www.R-project.org/
- 42.Hirsh Raccah B, Rottenstreich A, Zacks N. Appropriateness of direct oral anticoagulant dosing and its relation to drug levels in atrial fibrillation patients. J Thromb Thrombolysis. 2019;47(04):550–557. doi: 10.1007/s11239-019-01815-y. [DOI] [PubMed] [Google Scholar]
- 43.Comans A L, Sennesael A L, Bihin B, Regnier M, Mullier F, de Saint-Hubert M. Inappropriate low dosing of direct oral anticoagulants in older patients with non-valvular atrial fibrillation: impact on plasma drug levels. Thromb Res. 2021;201:139–142. doi: 10.1016/j.thromres.2021.02.034. [DOI] [PubMed] [Google Scholar]
- 44.Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators and Patients . Steinberg B A, Shrader P, Thomas L. Factors associated with non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with new-onset atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II) Am Heart J. 2017;189:40–47. doi: 10.1016/j.ahj.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 45.Yao X, Tangri N, Gersh B J. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70(21):2621–2632. doi: 10.1016/j.jacc.2017.09.1087. [DOI] [PubMed] [Google Scholar]
- 46.GARFIELD-AF Investigators . Camm A J, Cools F, Virdone S. Mortality in patients with atrial fibrillation receiving nonrecommended doses of direct oral anticoagulants. J Am Coll Cardiol. 2020;76(12):1425–1436. doi: 10.1016/j.jacc.2020.07.045. [DOI] [PubMed] [Google Scholar]
- 47.Sugrue A, Sanborn D, Amin M. Inappropriate dosing of direct oral anticoagulants in patients with atrial fibrillation. Am J Cardiol. 2021;144:52–59. doi: 10.1016/j.amjcard.2020.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roşian A N, Roşian ŞH, Kiss B. Interindividual variability of apixaban plasma concentrations: influence of clinical and genetic factors in a real-life cohort of atrial fibrillation patients. Genes (Basel) 2020;11(04):E438. doi: 10.3390/genes11040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raymond J, Imbert L, Cousin T. Pharmacogenetics of direct oral anticoagulants: a systematic review. J Pers Med. 2021;11(01):37. doi: 10.3390/jpm11010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueshima S, Hira D, Fujii R. Impact of ABCB1, ABCG2, and CYP3A5 polymorphisms on plasma trough concentrations of apixaban in Japanese patients with atrial fibrillation. Pharmacogenet Genomics. 2017;27(09):329–336. doi: 10.1097/FPC.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 51.Gouin-Thibault I, Delavenne X, Blanchard A. Interindividual variability in dabigatran and rivaroxaban exposure: contribution of ABCB1 genetic polymorphisms and interaction with clarithromycin. J Thromb Haemost. 2017;15(02):273–283. doi: 10.1111/jth.13577. [DOI] [PubMed] [Google Scholar]
- 52.Lähteenmäki J, Vuorinen A L, Pajula J. Pharmacogenetics of bleeding and thromboembolic events in direct oral anticoagulant users. Clin Pharmacol Ther. 2021;110(03):768–776. doi: 10.1002/cpt.2316. [DOI] [PubMed] [Google Scholar]
- 53.Gulilat M, Keller D, Linton B. Drug interactions and pharmacogenetic factors contribute to variation in apixaban concentration in atrial fibrillation patients in routine care. J Thromb Thrombolysis. 2020;49(02):294–303. doi: 10.1007/s11239-019-01962-2. [DOI] [PubMed] [Google Scholar]
- 54.Kreutz R, Persson P B, Kubitza D. Dissociation between the pharmacokinetics and pharmacodynamics of once-daily rivaroxaban and twice-daily apixaban: a randomized crossover study. J Thromb Haemost. 2017;15(10):2017–2028. doi: 10.1111/jth.13801. [DOI] [PubMed] [Google Scholar]
- 55.Artang R, Anderson M, Riley P, Nielsen J D. Assessment of the effect of direct oral anticoagulants dabigatran, rivaroxaban, and apixaban in healthy male volunteers using a thrombin generation assay. Res Pract Thromb Haemost. 2017;1(02):194–201. doi: 10.1002/rth2.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bertaggia-Calderara D, Kröll D, Gerschheimer C. Effect of rivaroxaban on thrombin generation in vivo. A study in obese patients. Int J Lab Hematol. 2018;40(01):e11–e14. doi: 10.1111/ijlh.12767. [DOI] [PubMed] [Google Scholar]
- 57.Siguret V, Abdoul J, Delavenne X. Rivaroxaban pharmacodynamics in healthy volunteers evaluated with thrombin generation and the active protein C system: modeling and assessing interindividual variability. J Thromb Haemost. 2019;17(10):1670–1682. doi: 10.1111/jth.14541. [DOI] [PubMed] [Google Scholar]
- 58.Sepúlveda C, Palomo I, Fuentes E. Primary and secondary haemostasis changes related to aging. Mech Ageing Dev. 2015;150:46–54. doi: 10.1016/j.mad.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 59.SAFIR study group . Hanon O, Vidal J S, Pisica-Donose G. Bleeding risk with rivaroxaban compared with vitamin K antagonists in patients aged 80 years or older with atrial fibrillation. Heart. 2021;107(17):1376–1382. doi: 10.1136/heartjnl-2020-317923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


