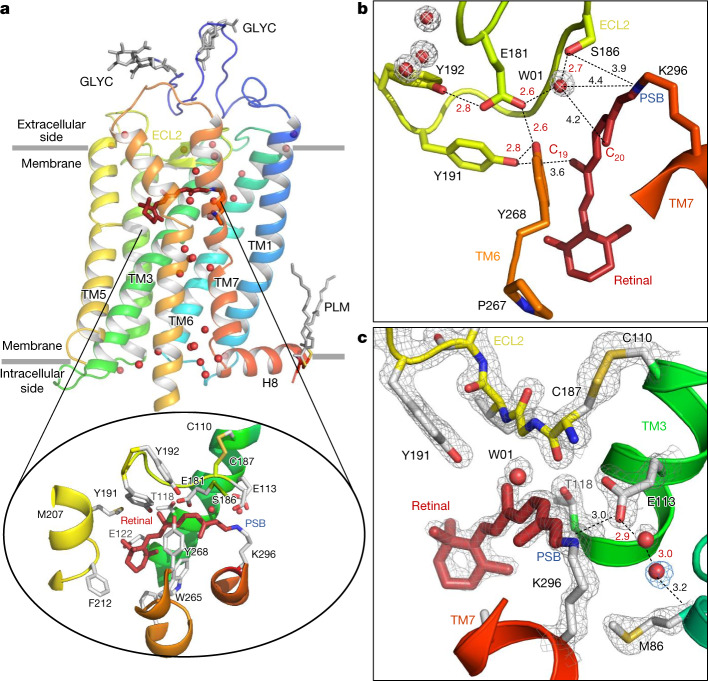
Fig. 1. Room temperature SFX structure of the dark state of bovine rhodopsin from crystals grown in LCP.
a, The overall structure of rhodopsin, rainbow coloured by residue number from blue (N terminus) to red (C terminus). The seven-TM bundle contains two N-glycosylation domains (GLYC) and palmitate groups (PLM) that anchor the amphipathic helix H8 to the membrane (grey lines). Water molecules (red spheres) form key networks1 between the extracellular (retinal ligand-binding pocket) and intracellular (G protein-binding site) regions of the receptor. The 11-cis retinal (dark red) is covalently bound to Lys296 (inset) through the PSB. The retinal-binding pocket is further composed of amino acids surrounding the PSB (the counterion Glu113, and Met44, Phe91, Thr94, Ala292 and Phe293), the retinal aliphatic chain (Ala117, Thr118, Tyr191, Trp265 and Glu181/Ser186 through water W01) and the β-ionone ring (Gly120, Gly121, Glu122, Phe212, Met207, Phe261 and Ala269; for clarity, only selected residues in the binding pocket are shown). b,c, Examples of well-resolved molecules in the water-mediated networks connecting the residues in the ancestral counterion Glu181 network (b) and the counterion Glu113 to Met86 of TM2 (and Ala1173.32, not shown) (c). The water molecules have well-defined electron densities (grey and blue meshes, 2Fobs − Fcalc electron density contoured at 2.2 and 0.7σ, respectively).