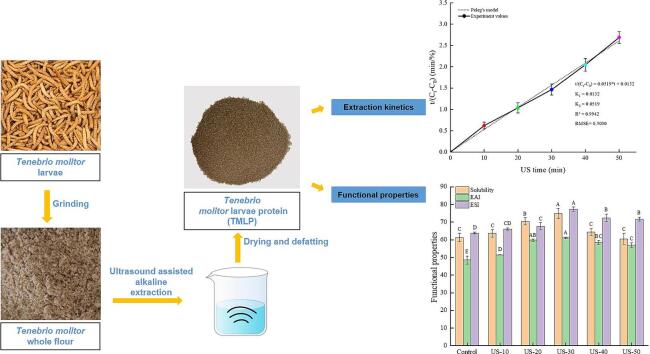
Graphical abstract
Keywords: Ultrasound assisted extraction, Extraction kinetics, Tenebrio molitor larvae protein, Secondary structures, Functional properties
Highlights
-
•
Ultrasound (US) was assisted to extract Tenebrio molitor larvae protein (TMLP).
-
•
US-assisted alkaline extraction enhanced the extraction yield of TMLP.
-
•
Peleg's model is a suitable model to represent the extraction kinetics of TMLP.
-
•
US altered the secondary structure, particle size and amino acid profiles of TMLP.
-
•
Functionalities of TMLP were enhanced by US-assisted alkaline extraction.
Abstract
Currently, as a promising alternative protein source, the interest of edible insect protein has been continuously increased. However, the extraction processing had distinct effects on the physicochemical properties and functionalities of this novel and sustainable protein. In this study, Tenebrio molitor larvae protein (TMLP) was extracted via ultrasound (US)-assisted alkaline extraction. The changes of extraction kinetics, physicochemical characteristics, and functional properties of TMLP as a function of US time (10, 20, 30, 40, 50 min) were investigated. The results showed that 30 min US treatment rendered the maximum protein yield (60.04 %) (P < 0.05). Meanwhile, Peleg's model was considered a suitable model to represent the extraction kinetics of TMLP, with a correlation coefficient of 0.9942. Moreover, the protein secondary structure, particle size, and amino acid profiles of TMLP were changed under the US-assisted alkaline extraction process. Additionally, a significant improvement of the functional properties of TMLP extracted with this method was observed compared to traditional alkaline extraction. In conclusion, the present work suggests that US-assisted alkaline extraction could be considered as a potential method to improve the protein yield, quality profiles, and functional properties of TMLP.
1. Introduction
In last decade, the continuously increasing challenges of population growth and climate changes are promoting the extensive search for sustainable and alternative protein sources for human beings [[1], [2]]. As indicated in a convey, some novel and sustainable proteins which originating from micro-/macroalgae, edible insects, microbes, fungi and leaves showed superior advantages than those which obtained from traditional sources, especially for their higher protein yield, superior techno-functionality, and greater nutritional values [3]. Recently, edible insects have attracted increasing interest among researchers and stakeholders in the industry, mainly due to their nutritional compounds, especially for proteins with adequate amounts of essential amino acids, lipids with a higher proportion of unsaturated fatty acids, and valuable vitamins (e.g. vitamin A, vitamin C, vitamin E, and niacin, etc.) and minerals (e.g. potassium, calcium, iron, magnesium, and zinc, etc.) [[4], [5]]. Moreover, grinding insects to powder could improve acceptability among wary consumers [6]. In the study of da Silva Lucasa, de Oliveira, da Rocha, and Prentice [7], they indicated that an increasing number of consumers preferred food products with ground edible insects rather than those presented with whole insects. Apart from its higher consumer acceptability, the processing of grinding or milling could significantly improve the physicochemical properties, volatile components, functional capacities, and the extraction rate of bioactive ingredients of the coarse edible insect flour [8]. However, the higher amount of lipids or insoluble chitin which included in edible insect flour were considered as huge obstacles for the potential application of edible insects in food processing, even for their excellent nutritional values [9]. Kim, Setyabrata, Lee, Jones, and Kim [10] also indicated the whole insect flour-added food products generally showed lower oxidative stability and in vitro digestibility which mainly attributed to the presence of unsaturated fatty acids and chitin. Thus, extraction processing and subsequent fractionation are considered crucial steps to use edible insects as sustainable protein source with excellent functional properties.
Among the various protein extraction strategies, the most conventional extraction method is firstly solubilized in alkaline solutions and subsequently recovered by using acid precipitation (the pH value ranged from 4.0 to 5.0) near the isoelectric point of edible insect protein [11]. Gravel and Doyen [12] also summarized that alkaline solubilization/isoelectric precipitation extraction showed significantly higher edible insect protein yield than other methods, such as aqueous (pH 7.0) extraction, micellar precipitation, organic solvents extraction, and salt extraction-dialysis. However, this conventional extraction strategy exhibited some issues, such as environmental pollution induced by acidic or alkaline wastewater, relatively longer extraction time, and higher solvent consumption [[13], [14]]. Moreover, Wang, Zhang, Xu, and Ma [15] indicated that the alkaline extraction/acid precipitation method could induce protein denaturation to some extent, resulting in quality deterioration and, consequently, limiting the application of the extracted proteins. Hence, some novel extraction technologies should be developed to assist the traditional extraction to shorten the extraction time and reduce energy consumption, as well as promote the yield and functionalities of target proteins.
Ultrasound (US) treatment, one of the most safe, efficient, green and economically feasible technology, has been commonly applied to assist to the extraction of proteins from various sources [[16], [17], [18], [19], [20], [21]]. Wu et al. [22] indicated that the higher protein yield of US-assisted alkaline extraction was mainly attributed to the fact that US could significantly enhance extraction efficiency by promoting mass transport and cell wall rupture induced by acoustic cavitation. Kadam, Tiwari, Álvarez, and O'Donnell [23] also indicated that the vibration induced by US could effectively promote the recovery of targeted compounds (especially proteins or bioactive peptides) from the solid raw material to the aqueous phase. However, the excessive ultrasound treatment could promote the formation of reactive free radicals under extreme vibration conditions, which subsequently led to serious protein or lipid oxidation [24]. Meanwhile, US-assisted alkaline extraction could alter the molecular structures of targeted proteins by destroying the hydrogen bonds or electrostatic forces through cavitation effect [25]. Xiong et al. [26] also indicated that US-assisted treatment could promote gelling properties, foaming capacities, emulsifying characteristics, and protein solubility. In this regard, US-assisted alkaline extraction has been widely studied in numerous previous works aiming to increase the yield and functionalities of target proteins from conventional plant- or animal-based sources [[1], [14], [16]]. Moreover, the extraction kinetics, which is expressed by a typical mathematical model, was considered as a substantial aspect to better understand the optimization, control, and design of the US-assisted extraction process [27]. Milićević et al. [28] indicated that Peleg's model was a typical and classical hyperbolic mathematical model originally aimed at predicting water sorption curves. Some previous studies have announced that Peleg's model could be commonly adapted to identify the best suitable parameters for the US-assisted extraction of rice bran oil [29], biflavonoids [30], and polyphenols [31]. However, few studies have been carried out to verify the suitability of Peleg's model in US-assisted alkaline extraction kinetics of edible insect proteins. To the best of our knowledge, previous studies mainly focused on the extraction of edible insect proteins by using traditional strategies. However, little information as regards to the effects of US-assisted alkaline extraction parameters (especially for US time) on the extraction kinetics, physicochemical properties and functional properties of edible insect proteins were available. Thus, the purpose of our present work was to study the effects of US-assisted alkaline extraction time (10, 20, 30, 40, 50 min) on the extraction yield, physiochemical characteristics (e.g., basic compositions, amino acid profiles, and secondary structures), and functional properties (e.g., solubility, emulsifying activity index, emulsifying stability index, water holding capacity, and oil holding capacity) of Tenebrio molitor larvae protein (TMLP). In addition, the extraction kinetics of TMLP obtained from the US-assisted alkaline extraction process was analyzed based using Peleg’s model. The obtained results will establish a novel US-assisted alkaline extraction method with higher extraction efficiency to produce TMLP, which could effectively and potentially promote the exploitation novel and sustainable protein with superior quality profiles.
2. Materials and methods
2.1. Insect samples
Living Tenebrio molitor larvae, which purchased from Zhongwang Breeding Co., ltd. (Xuzhou, Jiangsu, China), were transported to the lab keeping alive. Before protein extraction, all the impurities were separated by using a 20-mesh sieve.
2.2. US-assisted alkaline TMLP extraction
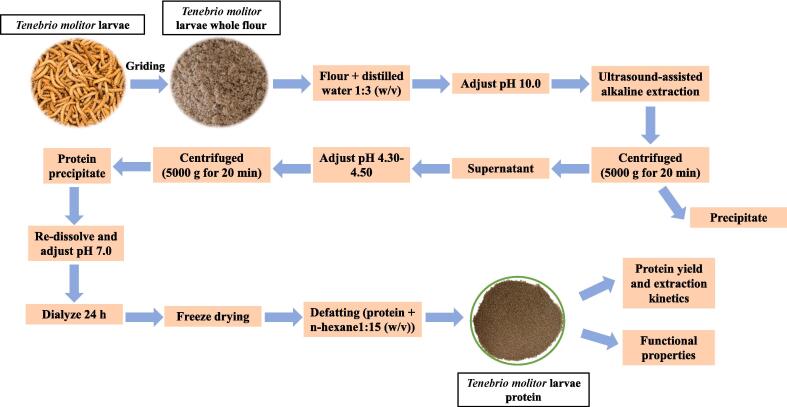
The US-assisted alkaline TMLP extraction procedures were according to the same method as described by Zhang et al. [32]. Briefly, the US-assisted alkaline extraction was carried out via an US processor (Scientz-50 T, NingBo Scientz Biotechnology Co. ltd., Ningbo, China). The US parameters were as follows: US power was 4 W/cm3, US frequency was 28 kHz, US temperature was kept at 25 ℃, US time was ranged from 10 to 50 min. The detailed US-assisted alkaline extraction flow chart is shown in Fig. 1.
Fig. 1.
Process flow chart for US-assisted alkaline extraction of TMLP.
2.3. Basic compositions
The basic compositions (e.g., moisture, protein, fat, and ash content) of the TMLP obtained from the US-assisted alkaline extraction under different US treatment times were determined using the method of Lenaerts et al. [33].
2.4. Protein yield
The protein yield of the TMLP obtained from the US-assisted alkaline extraction under different US time was calculated as follows:
where WP represents the weight of the TMLP, CP represents the concentration of the TMLP, WT represents the weight of the Tenebrio molitor larvae whole flours, and CTP represents the protein concentration of Tenebrio molitor larvae whole flours.
2.5. Extraction kinetics
The Peleg's model was used to describe the extraction kinetics of TMLP obtained from US-assisted alkaline extraction under different US treatment time. Peleg's model is a non-exponential model that was applied to explain the mass transfer rate in solid–liquid extraction and is generally used to illustrate the extraction curves of target components [[14], [27]]. Peleg's model equation is as follows:
| (1) |
where Ct is the extraction yield at time t (%), C0 is the initial extraction yield at time t = 0 (%), t (h) is the extraction time, K1 (min•%−1) and K2 (%−1) are Peleg's constants (rate constant and capacity constant, respectively).
According to this model, Equation (1) is transformed into Equation (2), where t is the independent variable and t/(Ct-C0) is the dependent variable, resulting in a linear equation. The intercept and slope of this equation are the rate constant K1 and the capacity constant K2.
| (2) |
The root mean square error (RMSE) was used to measure the goodness of fit of Peleg's model by comparing the experimental extraction yield with that calculated from TMLP, as follows:
| (3) |
2.6. Amino acid composition
The amino acid compositions of TMLP samples were measured according to the method of Kim et al. [34] and Yi et al. [9] via an amino acid analyzer (L-8800, Hitachi, Japan). Protein quality was expressed as the essential amino acid index (EAAI) and then calculated as follows:
The essential amino acids were used as the reference protein (Rf.) [35].
2.7. Determination of secondary structure
The secondary structures of TMLP obtained by US-assisted alkaline extraction under different US treatment times were determined using a circular dichroism spectrometer (CD, Chirascan, Applied Photophysics ltd., London, UK). The parameter settings were as follows: scanning rate was 100 nm/min, scanning band was from 190 to 260 nm, interval time was 0.25 s, slit width was 1.0 nm, and the minimum interval was 0.2 nm. The concentration of the TMLP solution was 0.25 mg/mL, and 10 mM phosphate-buffered saline (PBS) (pH 7.0) was used as a blank. All spectra were obtained with three sweeps. The secondary compositions of each TMLP sample (expressed as relative percentages of α-helix, β-sheet, β-turn, and random coil) were calculated using CD Pro. software.
2.8. The functional properties of TMLP
2.8.1. Particle size
The particle size of TMLP samples (concentration is 1.0 mg/mL) was measured according to the procedures of Jiang et al. [36].
2.8.2. Solubility
The TMLP was fully dispersed in 10 mM PBS (pH 7.0) to a final concentration of 10.0 mg/mL and subsequently centrifuged for 15 min at 8000 g under 4 °C. Protein concentrations of whole solutions and supernatants were measured according to Biuret method. The protein solubility was expressed as follows:
where Ps represents the protein concentration of the supernatant, and Pw represents the protein concentration of the whole solution.
2.8.3. Water holding capacity (WHC) and oil holding capacity (OHC)
The WHC and OHC were measured using the parameters of Zielińska et al. [6].
2.8.4. Emulsifying properties‘
The emulsification activity index (EAI) and emulsification stability index (ESI) of TMLP samples were determined using the procedures of Zhang, Xu et al. [5].
2.9. Statistical analysis
TMLP samples were prepared for three independent batches (replicates). For each batch of TMLP samples, determination of related traits were carried out in triplicate. All data was reported as the mean ± standard deviations (SD). The one-way analysis of variance (ANOVA) followed by Duncan's test was operated by using a Statistical Software (version 19.0, IBM SPSS Statistics, IBM., Chicago, USA) coupled with a statistical significance level of 0.05.
3. Results and discussion
3.1. The approximate composition
The approximate composition of TMLP is showed in Table 1. As shown in Table 1, with increasing of US treatment time, the protein contents of TMLP first increased and then decreased, reaching the maximum at 30 min (P < 0.05), which indicated that a moderate US treatment could improve the purity of TMLP. Meanwhile, when compared with control group, the US treatment had no significant effects on moisture contents (P > 0.05). However, the fat contents gradually increased with increasing of US treatment time (P < 0.05). This phenomenon was mainly due to that the prolonged US treatment probably promote the combination between protein molecules and lipid, which made it difficult to remove lipid in the defatting progress. Thus, the increased fat content as a function of US time (especially for longer than 30 min) might be the main reason for the decreased protein content. In addition, compared the control group, the ash contents decreased significantly with the US treatment (P < 0.05), which indicated that the insect shells were more easily separated from the TMLP with US treatment.
Table 1.
Effect of US time on the approximate compositions of TMLP which received from US-assisted alkaline extraction.
| Protein content (%) | Moisture content (%) | Fat content (%) | Ash content (%) | |
|---|---|---|---|---|
| Control | 75.75 ± 0.34C | 7.17 ± 0.67A | 6.95 ± 0.10C | 4.86 ± 0.42A |
| US-10 | 75.45 ± 0.16C | 7.22 ± 0.42A | 6.83 ± 0.06C | 4.27 ± 0.01B |
| US-20 | 76.89 ± 0.46B | 7.76 ± 0.14A | 7.46 ± 0.03B | 4.12 ± 0.06B |
| US-30 | 78.07 ± 1.26A | 7.75 ± 0.06A | 7.60 ± 0.28B | 4.04 ± 0.02B |
| US-40 | 75.48 ± 0.46C | 7.73 ± 0.08A | 8.54 ± 0.26A | 4.17 ± 0.01B |
| US-50 | 73.52 ± 0.33D | 7.72 ± 0.05A | 8.81 ± 0.15A | 4.25 ± 0.02B |
Values are given as means ± SD from triplicate determinations. Different letters (A-D) in the same column indicate significant differences (P < 0.05).
3.2. Protein yield and extraction kinetics
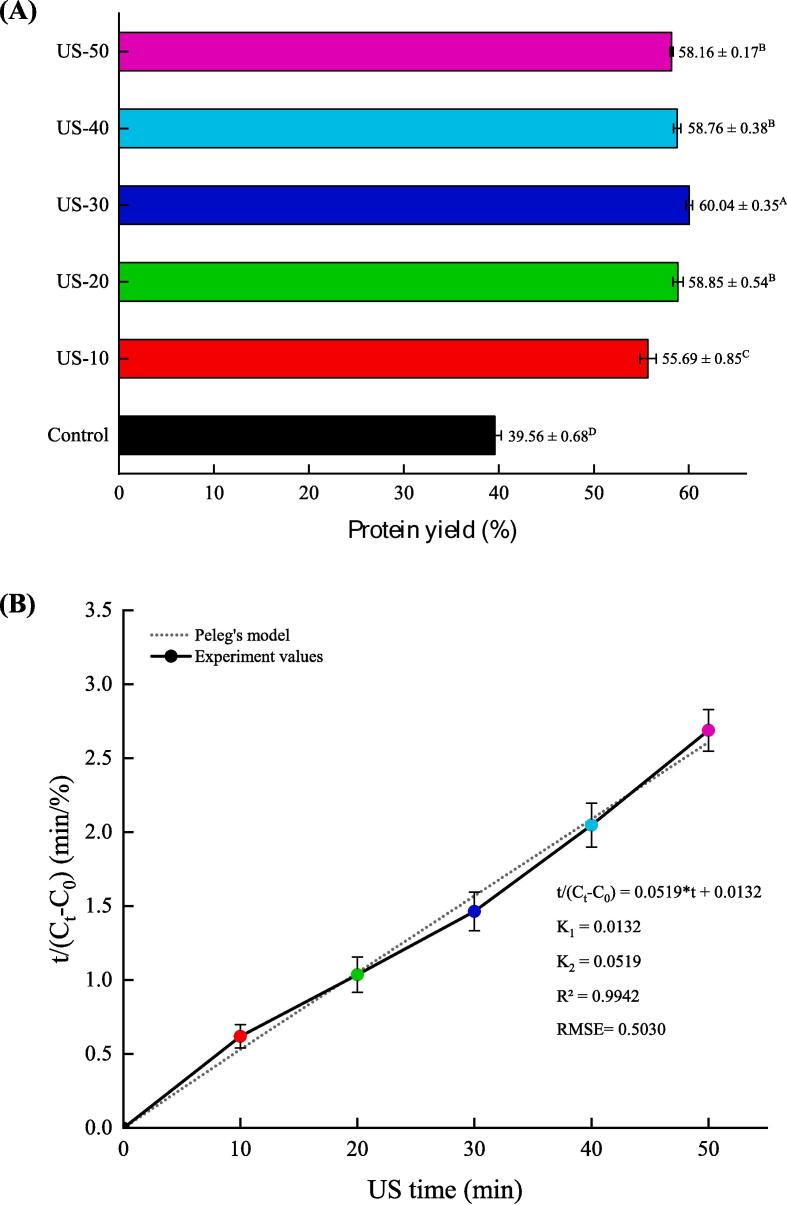
As shown in Fig. 2A, with increasing US treatment time, the protein yield increased and the maximum yield (60.04 ± 0.35 %) was obtained at 30 min (P < 0.05), which suggested that the protein yield can be improved with proper US treatment times. The main reason was that the physical, mechanical, and chemical effects induced by acoustic cavitation could improve the protein release rate [37]. However, there was a significant decrease in protein yield when the US treatment time was long. This decrease was possibly due to the reinforcement of the ultrasonic cavitation effect and due to the prolonged US treatment time, which possibly hindered the mass transfer effect, exposing and destroying the active site of the protein, thus reducing the protein yield [38]. Zhang, Wang, Zhang, Xu, and Hu [39] also found that US treatment for 30 min obtained the highest extraction yield of rice bran protein, and when the US treatment time was prolonged, the protein yield was slightly lower, probably because the long-term US treatment transformed the US energy into thermal energy, and the heat effect could lead to protein denaturation and insoluble. Additionally, the changes of protein yield as a function of US time showed a positive correlation with the change tendency of functional properties of TMLP.
Fig. 2.
Effect of US time on the protein yield (A) and extraction kinetics (B) of TMLP which received from US-assisted alkaline extraction. Values are given as means ± SD from triplicate determinations. Different letters (A-E) in the D4,3 indicate significant differences (P < 0.05).
During the extraction process, the protein yield (%) is greatly affected by the extraction method and parameters. In this study, to better demonstrate the influence of extraction methods and parameters on the extraction kinetics, non-exponential Peleg's model was used. As shown in Fig. 2B, the Peleg's equation curve of the US-assisted extraction was obtained with t as the horizontal coordinate and t/ (Ct-C0) as the vertical coordinate. Where, the intercept is the rate constant K1 and the slope of the line is the capacity constant K2. Currently, the Peleg's equation has been widely used to describe the extraction kinetics of various products with US-assisted extraction, such as rice bran oil, polysaccharides from mushroom by-products, polyphenols, and bitter melon seeds protein [[14], [27], [29], [31]]. In addition, Ge et al. [40] reported that the lower K1 and K2 values indicated the larger higher protein yield. In this paper, the K1 and K2 values were lower than the above research results, and the optimal US time was determined to be 30 min, based on the highest extraction yield of TMLP. Therefore, the optimal US-assisted extraction time was 30 min under the parameters of this experiment (4 W/cm3, 28 kHz). Furthermore, the Peleg's model values (the highest R2, 0.9942 > 0.95 and the lowest RMSE, 0.5030) showed a satisfactory agreement with the experimental values (Fig. 1B), which illustrating that the Peleg's model was able to explain the kinetics of US-assisted extraction of TMLP.
3.3. Amino acid composition and protein quality
The amino acid composition is shown in Table 2. Both contained lower levels of sulfur-containing amino acids, such as cysteine and methionine, which could be because they are destroyed during the alkaline extraction process for protein extraction [41]. In addition, the amino acid a composition showed that they are rich in aspartic acid and glutamic acid, indicating that the TMLP may possess acidic characteristics [42]. In Table 2, compared with the control group, the total amino acid content of all other groups was higher, and the US-30 group had the highest essential and non-essential amino acids, which indicated that some amino acids may be released during the US treatment process, leading to changes in the content and composition of amino acid [42]. The protein quality was usually evaluated by the essential amino acid index (EAAI), which was determined based on the nine essential amino acids (EAA) required to maintain animal health. As shown in Table 2, the EAAI value of all groups showed no obvious difference, which suggested that the US treatment did not negatively affect TMLP quality. In summary, the US is a feasible, quick, and simple auxiliary method to extract high quality TMLP that can be applied for multiple purposes.
Table 2.
Effect of US time on the amino acid profiles and protein quality of TMLP which received from US-assisted alkaline extraction.
| Unit (g/100 g protein) | Control | US-10 | US-20 | US-30 | US-40 | US-50 | Ref. |
|---|---|---|---|---|---|---|---|
| Essential amino acids (EAA) | |||||||
| Histidine (His) | 1.81 | 1.77 | 1.84 | 1.91 | 1.90 | 1.85 | 1.5 |
| Isoleucine (Ile) | 3.75 | 3.77 | 3.70 | 3.98 | 3.92 | 3.78 | 3.0 |
| Leucine (Leu) | 6.33 | 6.29 | 6.38 | 6.70 | 6.52 | 6.32 | 5.9 |
| Lysine (Lys) | 4.48 | 4.44 | 4.65 | 4.76 | 4.63 | 4.55 | 4.5 |
| Methionine + Cysteine (Met + Cys) | 2.06 | 2.03 | 1.92 | 2.12 | 2.04 | 2.13 | 2.2 |
| Phenyl-alanine + Tyrosine (Phe + Tyr) | 7.29 | 7.00 | 7.15 | 7.25 | 7.20 | 7.15 | 3.8 |
| Threonine (Thr) | 3.29 | 3.37 | 3.35 | 3.61 | 3.49 | 3.34 | 2.3 |
| Valine (Val) | 3.75 | 3.81 | 3.66 | 4.02 | 3.91 | 3.79 | 3.9 |
|
Sum of EAA Non-essential amino acids |
32.76 | 32.48 | 32.65 | 34.35 | 33.61 | 32.91 | 27.1 |
| Alanine (Ala) | 3.47 | 3.52 | 3.49 | 3.65 | 3.50 | 3.35 | |
| Arginine (Arg) | 3.84 | 3.89 | 4.09 | 4.18 | 4.04 | 3.98 | |
| Aspartic acid (Asp) | 6.96 | 7.09 | 7.24 | 7.69 | 7.46 | 7.26 | |
| Glutamic acid (Glu) | 9.04 | 9.08 | 9.77 | 9.97 | 9.32 | 9.18 | |
| Glycine (Gly) | 2.96 | 3.01 | 2.87 | 3.08 | 3.03 | 2.97 | |
| Proline (Pro) | 2.96 | 3.04 | 3.39 | 3.46 | 3.27 | 3.31 | |
| Serine (Ser) | 2.92 | 2.96 | 2.97 | 3.18 | 3.06 | 2.98 | |
| Sum of total AA | 64.91 | 65.07 | 66.47 | 69.56 | 67.29 | 65.94 | |
| EAAI | 1.87 | 1.86 | 1.88 | 1.89 | 1.88 | 1.87 |
Reference from FAO/WHO/UNU (1985).
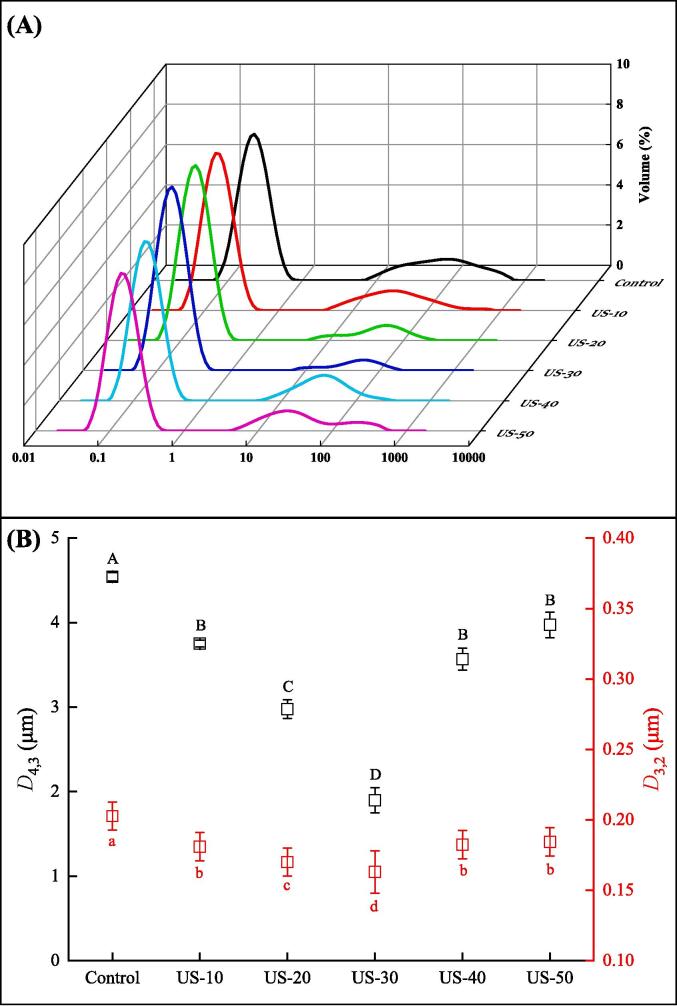
3.4. Particle size
Particle size has an important effect on the functional properties of proteins. The particle size distribution and average particle size of TMLP by different US time was shown in Fig. 3. As shown in Fig. 3A, there are two evident peaks can be observed at 0–1 μm and 1–1000 μm, respectively. At 0–1 μm, the peak shapes of TMLP samples from US groups were shifted to a smaller diameter than control group, indicating that a smaller particle size was obtained with US treatment. Jiang et al. [43] reported that ultrasonic treatment can destroy the non-covalent interaction of protein aggregates through the shear force generated by ultrasonic cavitation, allowing the particle diameter to be reduced. When at 1–1000 μm, compared with the control group, the particle size distribution of the other US-treated groups showed a narrowed peak, which suggested that the particle size distribution became more concentrated. In addition, similar results could be observed in the Fig. 3B. In Fig. 3B, the control group had the largest D (4, 3) and D (3, 2) values (P < 0.05). With increasing US treatment time, the D (4, 3) and D (3, 2) values first decreased and then increased, and the minimum of average particle size was obtained at 30 min (P < 0.05). Vargas et al. [44] found that US treatment decreased the particle size of whey protein, resulting from cavitation formed by the US treatment, as well as micro-streaming and turbulent forces. However, Gülseren, Güzey, Bruce, and Weiss [45] reported that when the US treatment time was longer than 40 min, the particle size of BSA increased, indicating that small aggregates may have been formed.
Fig. 3.
Effect of US time on the particle size distribution (A) and average diameter (B) of TMLP which received from US-assisted alkaline extraction. Values are given as means ± SD from triplicate determinations. Different letters (A-D) in the D4,3 indicate significant differences (P < 0.05). Different letters (a-d) in the D3,2 indicate significant differences (P < 0.05).
3.5. Secondary structure
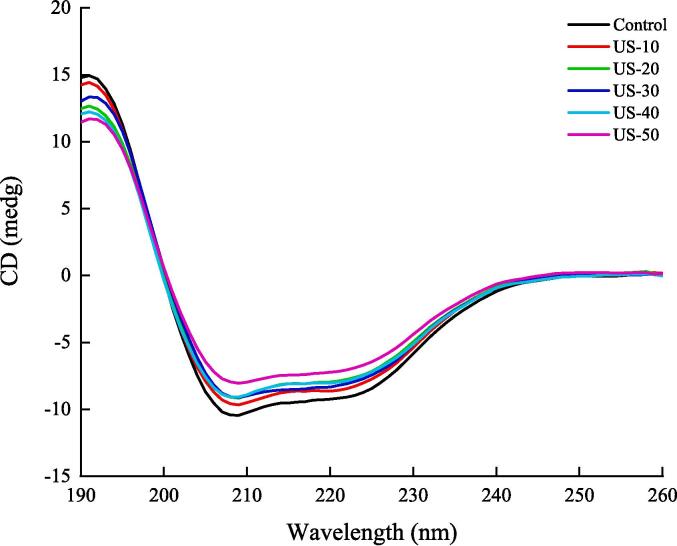
The use of CD to evaluate the secondary structure of TMLP extracted with different US treatment times is shown in Fig. 4. All samples had the similar CD spectra, with two negative peaks at 210 nm and 222 nm, which are indicative of a typical α-helix structure. Compared with the control group, the negative peak intensity of the TMLP extracted with different US treatment times increased, indicating that the α-helix content of the protein decreased, and the β-sheet content increased, which is consistent with the data in the Table 3. The changes of protein secondary structure is usually showed by ɑ-helix, β-sheet, β-turn and random coil contents. In Table 3, compared with the control group, with the increase of US time, the content of ɑ-helix and random coil decreased and the β-sheet contents increased (P < 0.05). It was probably because the ultrasound treatment caused the structure of TMLP to expand partially, and the hydrogen bond interaction is destroyed, resulting in the weakening of the rigid structure and the strengthening of the flexible structure of the protein, thereby resulting in the change of the secondary structure [15]. Zou et al. [41] obtained the similar results. They research the effect of US-assisted alkaline extraction on the secondary structure of chicken liver protein isolate and found that α-helix and random coil decreased, and in β-sheet contents increased.
Fig. 4.
Effect of US time on the circular dichroism of TMLP which received from US-assisted alkaline extraction.
Table 3.
Effect of US time on the secondary structure contents of TMLP which received from US-assisted alkaline extraction.
| α-helix (%) | β-sheet (%) | β-turns (%) | Random coil (%) | |
|---|---|---|---|---|
| Control | 19.46 ± 0.20A | 25.50 ± 0.34D | 19.90 ± 0.09A | 33.80 ± 0.17A |
| US-10 | 18.70 ± 0.10B | 26.66 ± 0.05C | 19.86 ± 0.05A | 33.30 ± 0.10B |
| US-20 | 18.20 ± 0.13C | 27.76 ± 0.07B | 19.53 ± 0.06B | 33.06 ± 0.11BC |
| US-30 | 17.59 ± 0.20D | 27.83 ± 0.25B | 19.96 ± 0.05A | 32.76 ± 0.21C |
| US-40 | 17.33 ± 0.05D | 28.06 ± 0.05B | 19.93 ± 0.05A | 32.13 ± 0.15D |
| US-50 | 16.43 ± 0.28E | 29.80 ± 0.43A | 19.59 ± 0.09B | 31.73 ± 0.23E |
Values are given as means ± SD from triplicate determinations. Different letters (A-E) in the same column indicate significant differences (P < 0.05).
3.6. The functional properties of TMLP
3.6.1. Solubility
The solubility is very important for evaluating their functional properties of proteins, which is positively correlated with protein particle size [46]. The solubility of TMLP for different US treatment times was shown in Table 4. With the increasing US traetment time, the solubility first increased and then decreased, and the maximum value was obtained at 30 min (P < 0.05). The cavitation bubbles produced by US can unfold proteins and reduce their particle size (Fig. 3B). Therefore, the protein conformation was modified, leading to more hydrophilic amino acid residues facing water, and improved interactions between protein and water, thereby increasing protein solubility [16]. However, with prolonged US time (>30 min), the solubility slightly decreased, which was possible due to the long-term US treatment could cause the formation of larger protein aggregates and then result in the decrease of solubility [47].
Table 4.
Effect of US time on the solubility, WHC, OHC, EAI, and ESI of TMLP which received from US-assisted alkaline extraction.
| Solubility (%) | WHC (g/g) | OHC (g/g) | EAI (m2/g) | ESI (%) | |
|---|---|---|---|---|---|
| Control | 61.35 ± 2.38C | 2.05 ± 0.10C | 1.48 ± 0.05C | 48.54 ± 2.31E | 63.82 ± 0.61D |
| US-10 | 63.66 ± 2.13C | 2.31 ± 0.39C | 1.49 ± 0.04C | 51.49 ± 0.16D | 66.10 ± 0.70CD |
| US-20 | 70.44 ± 2.04B | 2.94 ± 0.02B | 1.56 ± 0.03C | 59.77 ± 0.78AB | 67.64 ± 2.03C |
| US-30 | 74.92 ± 2.94A | 3.35 ± 0.07A | 1.82 ± 0.06A | 61.16 ± 0.40A | 77.43 ± 1.11A |
| US-40 | 64.38 ± 2.04C | 2.97 ± 0.05B | 1.71 ± 0.07B | 58.46 ± 1.25BC | 72.47 ± 2.01B |
| US-50 | 60.49 ± 3.13C | 2.02 ± 0.04C | 1.68 ± 0.02B | 57.08 ± 1.25C | 71.62 ± 1.06B |
Values are given as means ± SD from triplicate determinations. Different letters (A-E) in the same column indicate significant differences (P < 0.05).
3.6.2. WHC and OHC
WHC is a key attribute representing the water retention function, swelling, dissolution, and gel properties of proteins. Another key attribute is OHC, which indicates the ability of protein to absorb and retain fat. These two attributes are important in evaluating the texture and quality of products [48]. As shown in Table 4, with increasing US treatment time, the WHC first increased and then decreased, and the maximum value was obtained at 30 min (P < 0.05). The possible reason was that the US treatment can lead to the spongy structure of the peptide chain skeleton and the formation of some ionized polarity groups, leading to the formation of a loose structure and the improvement of the water binding capacity [41]. However, with the US time further prolonged (>30 min), under strong cavitation effect, the bubble collapse caused by US treatment could destroy both hydrophilic and hydrophobic groups, resulting in a lower WHC [49]. The OHC had similar results of the WHC. The aliphatic chains of the oil bind to the non-polar side chains of amino acids, which could result in the interaction of oil and protein, therefore, the higher hydrophobicity of protein, the better the OHC [17].
3.6.3. Emulsifying properties
The hydrophobic interaction is dominant at the oil–water interface, and the non-polar hydrophobic residue exposed at the interface has a great impact on the emulsification performance. The higher the surface hydrophobicity, the stronger the combination of emulsifier and oil droplet, and the better the emulsifying performance of protein [50]. As seen in Table 4, with increasing US treatment time, the EAI and ESI first increased and then decreased. In addition, compared with the control group, the EAI and ESI of all other US-treated groups significantly increased (P < 0.05), which indicated that the US treatment enhanced the emulsifying properties. The possible reason was that the US cavitation effect led to the unfolding of conformational structures of TMLP and more internal hydrophobic groups were exposed on the surface of TMLP, thus improving the interaction of oils and proteins [16]. In addition, the US treatment resulted in a reduction of the partial size of TMLP (Fig. 3B), and exposure of hydrophobic groups, thus resulting in stronger interaction of protein and oil and improved the emulsifying properties [51]. However, the prolonged US treatment (>30 min) caused a change in protein conformation and increased the content of insoluble protein aggregates, resulting in the decrease of EAI and ESI [17]. Moreover, under the prolonged US treatment, the strong US energy resulted in protein denaturation, which could influence protein migration and absorption at the oil–water interface, and then decrease the EAI and ESI.
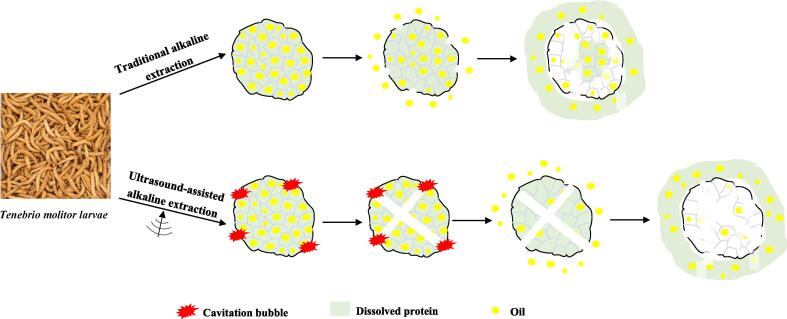
3.7. Mechanism of US-assisted extraction
Alkaline-solution and acid-isolation is the traditional approaches used for extract protein. The basic process is that the extracting solution is first exposed to an extremely alkaline pH and then adjusted back to isoelectric point. When the extracting solution is at an extreme alkaline pH enhanced charge repulsion moves the protein molecules to a partially unfolded state [1]. Subsequently, the acid precipitation steps recover the protein. However, this method cannot fully separate the protein, which can be observed in the Fig. 5. Therefore, the method of ultrasound assisted extraction is proposed. US-assisted extraction is an effective non-thermal physical extraction method for improving protein yield. The improved protein yield obtained by the US-assisted extraction is attributed to the cavitation effect generated in the solution by US wave [41]. As shown in Fig. 5, the high shear force and mechanical energy produced by US can cause cavitation, and the violent implosion of cavitation bubbles on the material surface can cause microjets, resulting in surface spalling, erosion and particle breakage [16]. These effects can lead to an increase in the solubility of proteins. Conversely, the enhanced US cavitation effect can cause cell wall disruption, and then result in the penetration of large amounts of solvent into the cellular material, improving mass transfer and releasing cellular contents, thereby promoting the dissolution of the protein [16].
Fig. 5.
Schematic mechanism of US-assisted alkaline extraction of TMLP.
4. Conclusion
The present work indicated that US-assisted alkaline extraction could be applied as a potential and effective extraction method to extract protein from Tenebrio molitor larvae. US processing time, a crucial extraction parameter, showed an significant enhancement on the extraction yield, physicochemical characteristics and functional properties of TMLP. The optimal US-assisted extraction time was 30 min under the US operating parameters of 4 W/cm3 and 28 kHz, with which the maximum protein yield of 60.04 % was obtained. Meanwhile, the Peleg's model was considered as the suitable model to represent the extraction kinetics of the TMLP, with a correlation coefficient of 0.9942. Moreover, the protein secondary structure, particle size, and amino acid profiles of TMLP were modified under the US-assisted alkaline extraction process. Meanwhile, a significant improvement of the functional properties of TMLP extracted with this method was observed compared to traditional alkaline extraction. Especially for 30 min US treatment, the TMLP had the highest solubility, water/oil holding capacities and emulsifying properties, which was positively correlated with the changes of protein yield. Therefore, the TMLP obtained by the US-assisted alkaline extraction processing could be acted as a sustainable source of protein which could be potentially applied in various food products. In future, we will investigate the effects of different parameters (e.g. frequency, power density, and temperature, etc.) of US-assisted alkaline extraction on the extraction kinetics, physicochemical characteristics, functionalities, and in vitro digestibility of TMLP, which could successfully establish a theoretical foundation for the application of TMLP in the food industry.
CRediT authorship contribution statement
Fengxue Zhang: Methodology, Investigation, Writing – original draft. Zhigang Sun: Investigation, Data curation. Xin Li: Software, Investigation, Validation. Baohua Kong: Data curation, Methodology. Fangda Sun: Visualization, Resources. Chuanai Cao: Software, Investigation, Validation. Qian Chen: Investigation, Data curation. Hongwei Zhang: Investigation, Methodology. Qian Liu: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the Natural Science Funds for Distinguished Young Scholars of Heilongjiang Province (Grant No. JQ2021C003) and National Natural Science Foundation of China (Grant No. 32172233).
Data availability
The data that has been used is confidential.
References
- 1.Alavi F., Chen L.Y., Emam-Djomeh Z. Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein. Food Chem. 2021;354 doi: 10.1016/j.foodchem.2021.129494. [DOI] [PubMed] [Google Scholar]
- 2.Zhang F.X., Cao C.A., Kong B.H., Sun F.D., Shen X.H., Yao X.Y., Liu Q. Pre-dried mealworm larvae flour could partially replace lean meat in frankfurters: Effect of pre-drying methods and replacement ratios. Meat Sci. 2022;188 doi: 10.1016/j.meatsci.2022.108802. [DOI] [PubMed] [Google Scholar]
- 3.Bedoya M.G., Montoya D.R., Tabilo-Munizaga G., Pérez-Won M., Lemus-Mondaca R. Promising perspectives on novel protein food sources combining artificial intelligence and 3D food printing for food industry. Trends Food Sci. Technol. 2022;128:38–52. doi: 10.1016/j.tifs.2022.05.013. [DOI] [Google Scholar]
- 4.Rumpold B.A., Schlüter O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013;57:802–823. doi: 10.1002/mnfr.201200735. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F.X., Xu Y.N., Kong B.H., Chen Q., Sun F.D., Zhang H.W., Liu Q. Comparative study of two types of pre-extraction treatment (drying or non-drying) on physicochemical, structural and functional properties of extracted insect proteins from Tenebrio molitor larvae. Curr. Res. Food Sci. 2022;5:1570–1580. doi: 10.1016/j.crfs.2022.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielińska E., Karaś M., Baraniak B. Comparison of functional properties of edible insects and protein preparations thereof, LWT-Food. Sci. Technol. 2018;91:168–174. doi: 10.1016/j.lwt.2018.01.058. [DOI] [Google Scholar]
- 7.da Silva Lucasa A.J., de Oliveira L.M., da Rocha M., Prentice C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020;311 doi: 10.1016/j.foodchem.2019.126022. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z.Q., Chen S.C., Wang Q.L., Liu C.Q., Xiao J.H., Huang D.W. Effects of traditional grinding and superfine grinding technologies on the properties and volatile components of Protaetia brevitarsis larvae powder. LWT-Food Sci. Technol. 2023;173 doi: 10.1016/j.lwt.2022.114307. [DOI] [Google Scholar]
- 9.Yi L.Y., Lakemond C.M.M., Sagis L.M.C., Eisner-Schadler V., Huis A.V., Boekel M.A.V. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013;141:3341–3348. doi: 10.1016/j.foodchem.2013.05.115. [DOI] [PubMed] [Google Scholar]
- 10.Kim H.W., Setyabrata D., Lee Y.J., Jones O.G., Kim Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016;38:116–123. doi: 10.1016/j.ifset.2016.09.023. [DOI] [Google Scholar]
- 11.Mishyna M., Martinez J.J.I., Chen J.S., Benjamin O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera) Food Res. Int. 2019;116:697–706. doi: 10.1016/j.foodres.2018.08.098. [DOI] [PubMed] [Google Scholar]
- 12.Gravel A., Doyen A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020;59 doi: 10.1016/j.ifset.2019.102272. [DOI] [Google Scholar]
- 13.Zou Y., Bian H., Li P.P., Sun Z.L., Sun C., Zhang M.H., Geng Z.M., Xu W.M., Wang D.Y. Optimization and physicochemical properties of nutritional protein isolate from pork liver with ultrasound-assisted alkaline extraction. Anim. Sci. J. 2018;89:456–466. doi: 10.1111/asj.12930. [DOI] [PubMed] [Google Scholar]
- 14.Naik M., Natarajan V., Modupalli N., Thangaraj S., Rawson A. Pulsed ultrasound assisted extraction of protein from defatted Bitter melon seeds (Momardica charantia L.) meal: Kinetics and quality measurements, LWT-Food. Sci. Technol. 2022;155 doi: 10.1016/j.lwt.2021.112997. [DOI] [Google Scholar]
- 15.Wang F., Zhang Y.Z., Xu L., Ma H.L. An efficient ultrasound-assisted extraction method of pea protein and its effect on protein functional properties and biological activities. LWT-Food Sci. Technol. 2020;127 doi: 10.1016/j.lwt.2020.109348. [DOI] [Google Scholar]
- 16.Wang Q., Wang Y., Huang M.G., Hayat K., Kurtz N.C., Wu X., Ahmad M., Zheng F.P. Ultrasound-assisted alkaline proteinase extraction enhances the yield of pecan protein and modifies its functional properties. Ultrason. Sonochem. 2021;80 doi: 10.1016/j.ultsonch.2021.105789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W.Y., Yang H.R., Coldea T.E., Zhao H.F. Modification of structural and functional characteristics of brewer’s spent grain protein by ultrasound assisted extraction. LWT-Food Sci. Technol. 2021;139 doi: 10.1016/j.lwt.2020.110582. [DOI] [Google Scholar]
- 18.Aadil R.M., Zeng X.A., Wang M.S., Liu Z.W., Han Z., Zhang Z.H., Hong J., Jabbar S. A potential of ultrasound on minerals, micro-organisms, phenolic compounds and colouring pigments of grapefruit juice. Int. J. Food. Sci. Tech. 2015;50:1144–1150. doi: 10.1111/ijfs.12767. [DOI] [Google Scholar]
- 19.Bhat Z.F., Morton J.D., Kumar S., Bhat H.F., Aadil R.M., Bekhit A.E.A. Ultrasonication as an emerging technology for processing of animal derived foods: A focus on in vitro protein digestibility. Trends. Food. Sci. Tech. 2022;124:309–322. doi: 10.1016/j.tifs.2022.04.012. [DOI] [Google Scholar]
- 20.Ranjha M.M.A.N., Irfan S., Lorenzo J.M., Shafique B., Kanwal R., Pateiro M., Arshad R.N., Wang L.F., Nayik G.A., Roobab U., Aadil R.M. Sonication, a potential technique for extraction of phytoconstituents: A systematic review. Processes. 2021;9:1406. doi: 10.3390/pr9081406. [DOI] [Google Scholar]
- 21.Chen J.H., Chen X., Zhou G.H., Xu X.L. New insights into the ultrasound impact on covalent reactions of myofibrillar protein. Ultrason. Sonochem. 2022;84 doi: 10.1016/j.ultsonch.2022.105973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu W.F., Jia J., Wen C.R., Yu C.P., Zhao Q., Hu J.N. Optimization of ultrasound assisted extraction of abalone viscera protein and its effect on the iron-chelating activity. Ultrason. Sonochem. 2021;77 doi: 10.1016/j.ultsonch.2021.105670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadam S.U., Tiwari B.K., Álvarez C., O'Donnell C.P. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci. Technol. 2015;46:60–67. doi: 10.1016/j.tifs.2015.07.012. [DOI] [Google Scholar]
- 24.Li Y.F., Zeng Q.H., Liu G., Peng Z.Y., Wang Y.X., Zhu Y.H., Liu H.Q., Zhao Y., Wang J.J. Effects of ultrasound-assisted basic electrolyzed water (BEW) extraction on structural and functional properties of Antarctic krill (Euphausia superba) proteins. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y.H., Cheng Y., Zhang Z.L., Wang Y., Mintah B.K., Dabbour M., Jiang H., He R.H., Ma H.L. Modification of rapeseed protein by ultrasound-assisted pH shift treatment: Ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrason. Sonochem. 2020;69:10524. doi: 10.1016/j.ultsonch.2020.105240. [DOI] [PubMed] [Google Scholar]
- 26.Xiong T., Xiong W.F., Ge M.T., Xia J.H., Li B., Chen Y.J. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018;109:260–267. doi: 10.1016/j.foodres.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 27.Aguiló-Aguayo I., Walton J., Viñas I., Tiwari B.K. Ultrasound assisted extraction of polysaccharides from mushroom by-products, LWT-Food. Sci. Technol. 2017;77:92–99. doi: 10.1016/j.lwt.2016.11.043. [DOI] [Google Scholar]
- 28.Milićević N., Kojić P., Sakač M., Mišan A., Kojić J., Perussello C., Banjac V., Pojić M., Tiwari B. Kinetic modelling of ultrasound-assisted extraction of phenolics from cereal brans. Ultrason. Sonochem. 2021;79 doi: 10.1016/j.ultsonch.2021.105761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.S. F. Garofalo, F. Demichelis, G. Mancini, T. Tommasi, D. Fino, Conventional and ultrasound-assisted extraction of rice bran oil with isopropanol as solvent, SUSTAIN. CHEM. PHARM. 29 (2022) 100741. https://doi.org/ 10.1016/j.scp.2022.100741.
- 30.L. Carrillo-Hormaza, L. Duque, S. López-Parra, E. Osorio, High-intensity ultrasound-assisted extraction of Garcinia madruno biflavonoids: Mechanism, kinetics, and productivity, Biochem. Eng. J. 161 (2020) 107676. https://doi.org/ 10.1016/j.bej.2020.107676.
- 31.Gisbert M., Sineiro J., Moreira M. Polyphenols extraction kinetics from Ascophyllum nodosum seaweed employing water and saltwater: Effect of ultrasound sonication. Algal Res. 2022;66 doi: 10.1016/j.algal.2022.102773. [DOI] [Google Scholar]
- 32.Zhang F.X., Yue Q., Li X., Kong B.H., Sun F.D., Cao C.A., Zhang H.W., Liu Q. Mechanisms underlying the effects of ultrasound-assisted alkaline extraction on the structural properties and in vitro digestibility of Tenebrio molitor larvae protein. Ultrason. Sonochem. 2023;94 doi: 10.1016/j.ultsonch.2023.106335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenaerts S., van der Borght M., Callens A., Campenhout L.V. Suitability of microwave drying for mealworms (Tenebrio molitor) as alternative to freeze drying: Impact on nutritional quality and colour. Food Chem. 2018;254:129–136. doi: 10.1016/j.foodchem.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Kim T.K., Yong H.I., Chun H.H., Lee M.A., Kim Y.B., Choi Y.S. Changes of amino acid composition and protein technical functionality of edible insects by extracting steps. J. Asia-Pac. Entomol. 2020;23:298–305. doi: 10.1016/j.aspen.2019.12.017. [DOI] [Google Scholar]
- 35.Fao, who, unu, Switzerland. p; Geneva: 1985. Energy and protein requirements: Report of a joint fao/who/unu expert consultation; p. 206. [Google Scholar]
- 36.Jiang S., Zhao S.C., Jia X.W., Wang H., Zhang H., Liu Q., Kong B.H. Thermal gelling properties and structural properties of myofibrillar protein including thermo-reversible and thermo-irreversible curdlan gels. Food Chem. 2020;311 doi: 10.1016/j.foodchem.2019.126018. [DOI] [PubMed] [Google Scholar]
- 37.Wen C.T., Zhang J.X., Yao H., Zhou J., Duan Y.Q., Zhang H.H., Ma H.L. Advances in renewable plant-derived protein source: the structure, physicochemical properties affected by ultrasonication. Ultrason. Sonochem. 2019;53:83–98. doi: 10.1016/j.ultsonch.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 38.Dong Z.Y., Li M.Y., Tian G., Zhang T.H., Ren H., Quek S.Y. Effects of ultrasonic pretreatment on the structure and functionality of chicken bone protein prepared by enzymatic method. Food Chem. 2019;299 doi: 10.1016/j.foodchem.2019.125103. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y.P., Wang B., Zhang W.N., Xu W., Hu Z.X. Effects and mechanism of dilute acid soaking with ultrasound pretreatment on rice bran protein extraction. J. Cereal Sci. 2019;87:318–324. doi: 10.1016/j.jcs.2019.04.018. [DOI] [Google Scholar]
- 40.Ge S.M., Tong X.Y., Gao C.F., Xu H.N., He R.H., Wu Q.Y., Wang J. Kinetics of silkworm pupae protein extraction at different ultrasonic frequency and temperature: Effects on physicochemical properties, functional properties and oxidation resistance. Process Biochem. 2022;122:36–52. doi: 10.1016/j.procbio.2022.09.025. [DOI] [Google Scholar]
- 41.Zou Y., Li P.P., Zhang K., Wang L., Zhang M.H., Sun Z.L., Sun C., Geng Z.M., Xu W.M., Wang D.Y. Effects of ultrasound-assisted alkaline extraction on the physiochemical and functional characteristics of chicken liver protein isolate. Poult. Sci. 2017;96:2975–2985. doi: 10.3382/ps/pex049. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y.J., Wen C.T., Feng Y.Q., Zhang J.X., He Y.Q., Duan Y.Q., Zhang H.H., Ma H.L. Effects of ultrasound-assisted extraction on the structural, functional and antioxidant properties of Dolichos lablab L. Protein. Process Biochem. 2021;101:274–284. doi: 10.1016/j.procbio.2020.11.027. [DOI] [Google Scholar]
- 43.Jiang S., Ding J., Andrade J., Rababah T.M., Almajwal A., Abulmeaty M.M., Feng H. Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments. Ultrason. Sonochem. 2017;38:835–842. doi: 10.1016/j.ultsonch.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 44.Vargas S.A., Delgado-Macuil R.J., Ruiz-Espinosa H., Rojas-Lopez M., Amador-Espejo G.G. High-intensity ultrasound pretreatment influence on whey protein isolate and its use on complex coacervation with kappa carrageenan: evaluation of selected functional properties. Ultrason. Sonochem. 2021;70:105340–105351. doi: 10.1016/j.ultsonch.2020.105340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gülseren I., Güzey D., Bruce B.D., Weiss J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007;14:173–183. doi: 10.1016/j.ultsonch.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Shi H.B., Zhou T., Wang X., Zou Y., Wang D.Y., Xu W.M. Effects of the structure and gel properties of myofibrillar protein on chicken breast quality treated with ultrasound-assisted potassium alginate. Food Chem. 2021;358 doi: 10.1016/j.foodchem.2021.129873. [DOI] [PubMed] [Google Scholar]
- 47.Maity I., Rasale D.B., Das A.K. Sonication induced peptide-appended bolaamphiphile hydrogels for in situ generation and catalytic activity of Pt nanoparticles. Soft Matter. 2012;8:5301. doi: 10.1039/c2sm25126d. [DOI] [Google Scholar]
- 48.Foegeding E.A., Davis J.P. Food protein functionality: A comprehensive approach. Food Hydrocoll. 2011;25:1853–1864. doi: 10.1016/j.foodhyd.2011.05.008. [DOI] [Google Scholar]
- 49.Zupanc M., Pandur Ž., Perdih T.S., Stopar D., Petkovšek M., Dular M. Effects of cavitation on different microorganisms: The current understanding of the mechanisms taking place behind the phenomenon. A review and proposals for further research. Ultrason. Sonochem. 2019;57:147–165. doi: 10.1016/j.ultsonch.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Gong K.J., Shi A.M., Liu H.Z.L., Hu H., Adhikari B., Wang Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016;170:33–40. doi: 10.1016/j.jfoodeng.2015.09.011. [DOI] [Google Scholar]
- 51.O’Sullivan J., Park M., Beevers J. The effect of ultrasound upon the physicochemical and emulsifying properties of wheat and soy protein isolates. J. Cereal Sci. 2016;69:77–84. doi: 10.1016/j.jcs.2016.02.013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.