Summary
Monodomain liquid crystal elastomers (m-LCEs) exhibit large reversible deformations when subjected to light and heat stimuli. Herein, we developed a new method for the large-scale continuous preparation of m-LCE fibers. These m-LCE fibers exhibit a reversible contraction ratio of 55.6%, breaking strength of 162 MPa (withstanding a load of 1 million times its weight), and maximum output power density of 1250 J/kg, surpassing those of previously reported m-LCEs. These excellent mechanical properties are mainly attributed to the formation of a homogeneous molecular network. Furthermore, the fabrication of m-LCEs with permanent plasticity using m-LCEs with impermanent instability without external intervention was realized by the synergistic effects of the self-restraint of mesogens and the prolonged relaxation process of LCEs. The designed LCE fibers, which are similar to biological muscle fibers and can be easily integrated, exhibit broad application prospects in artificial muscles, soft robots, and micromechanical systems.
Subject areas: Bionics, Materials processing, Mechanical property
Graphical abstract

Highlights
-
•
Monodomain liquid crystal elastomer (m-LCE) with permanent plasticity was prepared
-
•
A new method for the large-scale preparation of m-LCE fibers is presented
-
•
M-LCE fibers have superior actuation performance compared to skeletal muscle fibers
Bionics; Materials processing; Mechanical property
Introduction
Bionic smart fibers with shape memory characteristics have significant application potential in advanced manufacturing,1 medical devices,2 bionic structures,3 and space flight.4 Compared to shape-memory alloys,4 shape-memory polymer fibers (SMPFs) exhibit low densities, large deformations, adjustable phase-transition temperatures, and wider potential applicability. The shape memory property of an SMPF is generally irreversible. Recent studies have demonstrated that reversible shape-memory polymer fibers (RSMPFs) can be prepared via the induced directional crystallization of crystalline polymers in the polymer network.5,6,7 Monodomain main-chain liquid crystal elastomers (m-LCEs) can exhibit reversible deformation of more than 40% along the macroscopic arrangement orientation of the mesogens when heated. In this case, the directional arrangement of the mesogens and the fixation of the molecular alignment network are important processes. m-LCEs are mainly prepared using the "one-step method",8,9 "two-step cross-linking method",10,11 and reversible dynamic covalent bond exchange.12,13 The "one-step method" generally utilizes anchoring energy from the surface8 and an electric or magnetic field9 to achieve the directional arrangement of the molten mesogens in the liquid crystal cell; a light or heat initiator is then used to form a small micron-thickness film sample. The "two-step cross-linking method" is widely utilized to prepare long-term stable m-LCEs with excellent reversible shape memory characteristics. The lightly cross-linked network with excessive mesogens is stretched mechanically, and then, the directional arrangement of mesogens is fixed by polymerizing the free liquid crystal end groups (acrylate groups). The shear stress during the extrusion molding process is used to achieve a certain degree of directional arrangement of the mesogens and is combined with light curing for preparing LCE fibers.14,15,16,17
Generally, the high strength of the fibers is an essential requirement for their effective applications. The "two-step cross-linking method" involves the mechanical stretching (150%–200%) and secondary cross-linking curing process of the m-LCEs. During these processes, the fixation of the high elastic deformation of the partially cross-linked network and the synthesis of side-chain liquid crystal structures by acrylate groups can severely disrupt the homogeneity of the liquid crystal network and consequently affect the mechanical properties, such as its breaking strength. Currently, LCEs with single-molecule networks generally have inferior mechanical properties than interpenetrating LCEs. Their elastic modulus and maximum stress are in the range of 0.5–1.4 MPa, when the temperature is above the anisotropic-isotropic transition threshold (Tni).18,19,20,21,22,23,24,25,26 Therefore, m-LCEs should be combined with polymer networks possessing high moduli,27 which requires a complicated process flow, thereby limiting their applications.
In this study, a liquid crystal elastomer bionic muscle fiber (LCF) with a high modulus and controllable diameter was prepared directly and continuously by the liquid-phase drawing method at room temperature. This approach does not involve light/heat curing and dynamic covalent bond exchange reactions. The axial alignment of mesogens in monodomain LCFs (m-LCFs) is determined by the self-restraint of the mesogens and polymer relaxation. This method can be extended to continuous m-LCE films. In addition, owing to the maximum actuated load stress of 5.35 MPa, the mechanical properties and actuation performance of m-LCF are considerably superior to those of the smallest functional unit of biological muscle contraction (muscle fiber). We constructed lightweight bionic muscles from the m-LCF bundles and demonstrated their actuation. This study improves the actuation performance of LCEs and extends their possible applications.
Results and discussion
Fabrication of m-LCF
Figure 1A shows a schematic of the liquid-phase fiber-drawing method for preparing m-LCFs and the microscopic molecular network change process. The liquid crystal prepolymer was first formed through a sealed thiol-ene click reaction between the liquid crystal monomer and flexible chain extender (molar ratio = 1:0.85) with an appropriate amount of tetrahydrofuran (THF) at 25°C. Afterward, in the presence of the cross-linking agent, pentaerythritol tetra(3-mercaptopropionate) (PETMP), with a mesogens: PETEP molar ratio of 1:0.075, the molecular weight of the prepolymer and the solution viscosity continue to increase (Figure S1). The acrylate and thiol groups are equal in the prepolymer. The prepolymer gradually forms a loose cross-linked network. When the viscosity of prepolymer increased to approximately 13.5 Pa s (2,2′-oxydiethanethiol (DSH)-LCEs), an LCF with a uniform diameter can be directly and continuously drawn vertically using a metal tractor from an elasto-viscous fluid. In this case, the elasto-viscous prepolymer solution containing macromolecular polymers adhered to the traction head can overcome anelasticity and cohesion when forming a new surface under the action of traction, eventually deforming into fibers. Figure S2 shows an image of the fiber-drawing process of the LCF, which is demonstrated in Video S1. The combination of the rapid solvent volatilization and continuous cross-linking of the prepolymer allowed the curing and continuous preparation of the fibers. The fiber-drawing equipment comprised a controllable base slider, drawing roller, and heat source. The space between the base slider and the drawing roller was vertical. A uniform large-scale preparation of LCFs can be achieved by the coordinated control of the roller rotation and base translation speeds. After maturation at 40–45°C for 48 h, the fibers were stretched and collected into rolls and stored for 20 d. The mechanism of the alignment of mesogens is discussed in section “the alignment mechanism of m-LCF”.
Figure 1.
The preparation process and microscopic photographs of m-LCF
(A) Schematic of the continuous preparation process of liquid crystal elastomer bionic muscle fiber (LCF) using the liquid-phase fiber-drawing method. Ⅰ, Ⅱ, and Ⅲ show the three changing stages of the liquid crystal molecular network during the monodomain LCF (m-LCF)-forming process; the upper left corner is the molecular chain structure of RM257; the upper right corner is the image of the fiber roll of DSH-m-LCF and the two-dimensional wide-angle X-ray diffraction (2D-WAXD) of the fiber.
(B) Scanning electron microscopy (SEM) images of DSH-m-LCF with different diameters.
Figure 1A shows the three changing stages, I, II, and III, of the liquid crystal molecular network during the m-LCF-forming process. Due to the volatilization of the solvent, the distance between the liquid crystal molecules. In stage III, the mesogens achieved permanent alignment along the fiber axis owing to the interaction force of mesogens induced by slight stress combined with the prolonged relaxation process. The right panel of stage III (Figure 1A) shows the prepared fiber roll of DSH-m-LCF and its two-dimensional wide-angle X-ray diffraction (2D-WAXD) pattern. The corresponding order parameter was 0.72. In addition, its reversible contraction ratio reached 55.6% on a hot stage, which is significantly higher than that of reported m-LCEs with the same material system. The reversible contraction ratio of DSH-m-LCF did not change significantly within 500 cycles, as shown in Figure S3A, indicating that the prepared m-LCF had a good cycle stability. To eliminate the friction between the hot stage and m-LCF, we measured the contraction ratio of the m-LCE fiber in the temperature chamber. A small load (20 mg) was suspended at the end of the fiber to prevent fiber curling. The contraction ratio was 55.6%. The test image is shown in Figure S3B. The contraction ratio and order parameters revealed that the mesogens in the m-LCF were perfectly aligned. Figure S4 shows an illustration of the customized sewing and bonding of DSH-m-LCF fibers on the fabric, with more details shown in Video S2.
Figure S5 shows the variation in the axial tensile force under the joint action of the internal friction force and self-weight of the fiber during the drawing process. As indicated by the slope of the axial tension curve in the fiber-drawing process, the regulation of the lifting speed can afford the preparation of a relatively uniform fiber. The change in the axial tensile force during the fiber-drawing process is shown in Video S3. Furthermore, the influence of the drawing speed on the fiber formation was examined through the change in the axial tensile force. Further information is provided in Figure S6A. In addition, the diameter of the fiber was controlled by selecting the diameter of the traction head. Figure S6B shows the relationship between the diameter of the m-LCF drawn at a speed of 30 cm/min and the outer diameter of the traction head. This demonstrates that R(Traction rod)/R(Fiber) approximately satisfies Equation 1.
| (Equation 1) |
This enables the controlled preparation of m-LCFs with different diameters. Figure 1B shows the scanning electron microscopy (SEM) images of DSH-m-LCFs with different diameters (0.2–0.5 mm). The diameters of fibers are uniform and exhibit a smooth surface. Mechanical tests revealed that the diameter of the DSH-m-LCF had negligible effects on its mechanical properties. Overall, the strength increases as the diameter decreases (Figure S7). The drawing speed of the LCF described below was 30 cm/min.
Mechanical property analysis of m-LCF
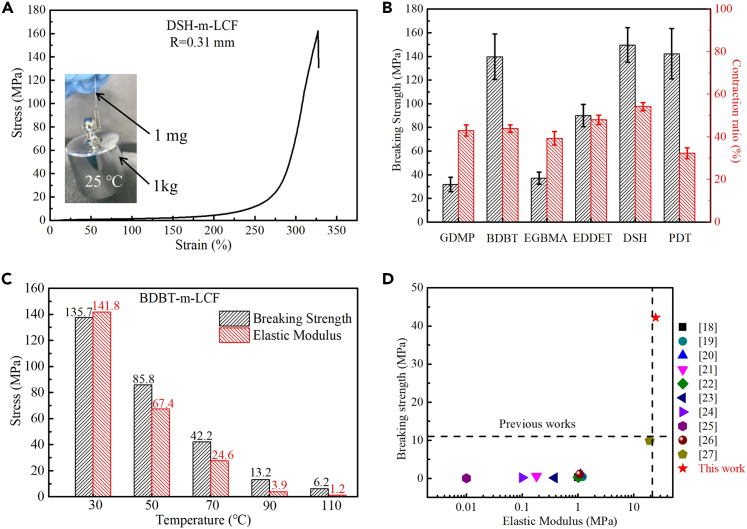
The reported breaking strength of m-LCEs based on the acrylate-sulfhydryl reaction was 3–14 MPa at room temperature. The inset in Figure 2A shows a DSH-m-LCF with a mass of approximately 1 mg withstanding a weight of 1 kg (1 million times its weight) at room temperature. Its breaking strength reaches a maximum of 162 MPa, which is two orders of magnitude higher than the previously reported values. m-LCEs with a lower proportion of mesogens and higher flexibility of the main chain have a lower phase-transition temperature and reversible contraction ratio.28 However, the factors affecting their mechanical properties remain unknown. Significantly, the flexibility of a molecular chain can change its conformation.
Figure 2.
The mechanical property of different m-LCF at different temperatures
(A) Stress-strain curve of DSH-monodomain liquid crystal elastomer bionic muscle fiber (m-LCF). Inset graph shows a DSH-m-LCF with a mass of ∼1 mg withstanding a 1 kg weight (1 million times its weight) at room temperature.
(B) Histogram of the breaking strength and the reversible deformation performance of the six m-LCFs.
(C) Histogram of the breaking strength and elastic modulus of BDBT-m-LCF at different temperatures.
(D) Ashby-like plot comparing the breaking strength and elastic modulus of BDBT-m-LCF in this study and reported related data at the temperature above anisotropic-isotropic transition threshold (Tni).
We systematically investigated the effect of different groups in different linear dithiols (unbranched) on m-LCFs using 2-Methyl-1,4-phenylene bis(4-(3-(acryloyloxy)propoxy)benzoate) (RM257) as the mesogens, dithiols as the chain extender, and PETMP as the cross-linking agent. The structural formulas of all the chain extenders are shown in Figure S8. m-LCFs with ethylene glycol bis(3-mercaptopropionate) (GDMP), 1,4-butanediol bis(mercapto ester (BDBT), bis(thioglycolate) glycol ester (EGBMA), 3,6-dioxa-1,8-octanedithiol (EDDET), DSH, and 1,3-propanedithiol (PDT) as chain extenders have been extensively studied. Figure 2B compares the breaking strengths and reversible deformation performances of the six m-LCFs. The differential scanning calorimetry (DSC) data of the m-LCFs (Figure S9), the effect of the cross-linking agent content (Figure S10), and the mechanical properties of several long straight-chain alkyl dithiols (Figure S11) are presented in the Supporting Information.
The differences in the mechanical properties of m-LCFs with GDMP, BDBT, and EGBMA as chain extenders were noticeable. Comparing the structural formulas of the three chain extenders, the issues originate from the difference in the number of alkyl groups between the two ester groups, which leads to considerable differences in the overall mechanical properties of m-LCFs. The degree of entanglement of the chain segment was higher when fewer alkanes were present between the ester groups, resulting in an abrupt increase in stress points during stretching and a rapid deterioration of mechanical properties. The same trend is observed for EDDET-containing "–O–(CH2)2–O–" segments. Although the ether-based chain was more flexible than that of the ester group, the low segment length improved the overall rigidity and ensured the optimal mechanical properties of the fiber. Chain extenders with "–COO–(CH2)4–COO–", "–O–", or alkane chains exhibited excellent rigidity, resulting in an ultrahigh breaking strength of >150 MPa for BDBT-m-LCF, DSH-m-LCF, and PDT-m-LCF. Although increasing the rigidity of the flexible chain extender benefits the overall fiber mechanical properties, the energy required for the rotation of the liquid crystal molecules correspondingly increases, implying that the actuation temperature increases. This study accounts for the effects of the segmented length of the chain extender and the number and location of ester and ether groups on Tni as well as the reversible deformation performance of LCEs. Therefore, it can provide some reference for the research on the mechanical properties of LCEs.
In addition, the presence of a side-chain liquid crystal structure polymerized by two or more acrylate groups could be a reason for the poor mechanical properties of the traditionally light-cured LCEs. This disrupts the homogeneity of the molecular network (Figure S12), deteriorating its macroscopic mechanical properties. Using EDDET-LCEs as an example, Figure S13 compares the properties of the LCE films, which underwent the second light-curing process, without pre-stretching and with different excessive acrylate contents, and those with different pre-stretching treatments with 10% excessive acrylate. The adverse effects of the side-chain liquid crystal structure produced via the polymerization of acrylate groups on the LCEs were revealed from Figure S13. Furthermore, a large pre-stretching rate is essential for m-LCEs prepared via the "two-step cross-linking method," which leads to an ideal reversible deformation. However, it causes a significant slippage of the uncross-linked segments and increases the highly elastic deformation of the cross-linked network. This necessitates an unavoidable trade-off between reversible deformation and mechanical properties.29 The liquid-phase fiber-drawing method proposed in this article eliminates acrylate group polymerization. Moreover, it significantly reduces the defects in the molecular network, which improves the mechanical properties while achieving a high reversible contraction ratio. Another important reason for the excellent mechanical properties of m-LCF is the structural superiority of the one-dimensional fibers. Furthermore, the highly directional arrangement of mesogens allows for the tightest stacking of the liquid crystal molecules in the fiber, reducing the defects and significantly improving its mechanical properties.
BDBT-m-LCF exhibited a breaking strength of 42.2 MPa at temperatures higher than Tni (70°C), which is significantly higher than other previously reported values.18,19,20,21,22,23,24,25,26,27 Figure 2C depicts the breaking strength and elastic modulus of BDBT-m-LCF at various temperatures and Figure 2D compares the results of the present study to previously reported relevant data at temperatures above Tni.
Actuation performance of LCF and actuation demonstration
The actuation performance of m-LCF was significantly higher than that of the currently reported m-LCEs, primarily because of the high degree of directional arrangement of mesogens and excellent mechanical properties. The m-LCFs were actuated via thermal convection. Figure 3A shows a schematic of the actuation test of the m-LCF under different loads. High- and low-temperature chambers were utilized to change the temperature, and a thermocouple and high-definition camera recorded the temperature and length of the m-LCF in real-time. Figure 3B shows the temperature-deformation curve of DSH-m-LCF under a load of 0.1 MPa, where the reversible contraction ratio reaches 55.4%. The change in ΔL/L(stretch) of DSH-m-LCF under different loads at 130°C is shown in Figure 3C. Figure S14 demonstrates the actuation contraction process of a 0.94 mg DSH-m-LCF (r = 0.19 mm) with a load of 60 g (equivalent to 60,000 times its weight). The actuated load stress reaches 5.34 MPa, and the output work density is 1250 J/kg, which is significantly higher than that of previously reported m-LCEs. In addition, the generated contractile force of DSH-m-LCF was measured along with the temperature variation using a thermomechanical analyzer (TMA) in the isostrain mode, as shown in Figure 3D. Figures 3E and 3F show the variation curve of the contractile force in the range of 20–130°C, where the initial load stress is 0 and 4 MPa, respectively, demonstrating that m-LCF exhibits a good contractile force.
Figure 3.
The actuation performance and actuation demonstration of m-LCF
(A) Schematic of the actuation strain test of the m-LCF under different loads.
(B) Temperature-deformation curve of DSH-m-LCF under a load of 0.1 MPa.
(C) ΔL/L (stretch) of DSH-m-LCF under different loads at 130°C.
(D) Schematic of the measure of the contractile force using the thermal-mechanical analyzer (TMA) in the isostrain mode.
(E) Variation curve of the contractile force of DSH-m-LCF with 0 MPa load stress.
(F) Variation curve of the contractile force of DSH-m-LCF with 4 MPa load stress.
(G) Actuation demonstration of the spindle-shaped m-LCF artificial muscle under thermal stimulation and the corresponding molecular network change process.
Based on the excellent thermo-drive performance of DSH-m-LCF, a bionic artificial muscle was used to simulate biological muscles. Figure 3G shows an image of certain m-LCFs as muscle bundles that lift heavy objects under thermal convection. The m-LCFs were remotely heated using carbon fiber heating tubes. Three-dimensional-printed gear sheets separated 20 m-LCFs, and each m-LCF had a groove to facilitate uniform heating and rapid heat dissipation during the actuation process. The schematic of the printed structures is shown in Figure S15. The spindle-shaped m-LCF muscle bundle was 15 cm long and could achieve a contraction of approximately 34% under a load of 233 g. The actuation of the m-LCF artificial muscle is shown in Video S4.
The alignment mechanism of m-LCF
Considering the EDDET-LCE system as an example, the reversible contraction ratio of EDDET-m-LCF (47% ± 2.2%) was higher than the reported value.10,11 This demonstrates the suitability of the order parameter of the mesogens in m-LCF prepared via the liquid-phase fiber-drawing method. The fiber-drawing process is similar to the well-known wire-drawing process. When a tangential force is applied to a viscous polymer fluid (relaxation time, τ >> 0), the generated axial velocity gradient promotes the extension of the winding and curling polymer molecular chain and the alignment of the macromolecule along the fiber axis. Figure S16 shows the simulation diagram of the flow velocity of different fluid layers at the root of the fiber during the drawing process of the viscous fluid simulated using the COMSOL software. An inevitable speed gradient was observed in the axial and radial directions during the drawing process of the liquid crystal prepolymer. However, the degree of directional arrangement caused by the speed gradient in the drawing process is generally minimum. The fabricated LCF that was left for 360 h did not exhibit actuation performance. The 2D-WAXD image is shown in Figure S17A, and the corresponding order parameter is 0.14. However, when a 5 cm-long LCF was stretched to 300% and released, its length reached 8.2 cm (approximately 64% elongation). In addition, it achieves 100% reversible deformation at temperatures below 55°C, as shown in Figure 4A. The 2D-WAXD image of the LCF after stretching (Figure S17B) reveals that the mesogens are aligned along the axial direction with an order parameter of 0.64. However, the directional arrangement of mesogens and the reversible actuation performance of the LCF rapidly disappeared above 60°C. The experiment demonstrated that the stress-induced interaction force between the directionally aligned molecules retained the mesogens in the alignment state after relaxing the exterior stress.
Figure 4.
The alignment mechanism of m-LCF
(A) Length of 5 cm-long LCF obtained with different treatments and temperatures. Fiber was loaded with 0 kPa within 360 h.
(B) Simulation diagram of two molecules with a dipole moment of 3.40 D.
(C) Length as a function of time at different stresses of the horizontally placed nascent 1,4-butanediol bis(mercapto ester (BDBT)-LCF prepared via the liquid-phase fiber-drawing method.
(D) Ratio of the mass of residual solvent in the LCF to the mass of completely desolvated LCF at different times after the fiber-drawing process, and the molar ratio of solvent molecules to mesogens.
(E) Length change in each interval (5 cm/each interval) of the nascent BDBT-LCF with a load of 10 kPa for different hanging times.
Thermotropic liquid crystals in the molten state can maintain high-order parameters. However, their directional arrangement can be destroyed as the distance between the molecules increases when dissolved in the solution. The RM257 liquid crystal monomer is a typical rod-shaped polar molecule. We constructed more than 20 initial RM257 structures and used density functional theory to optimize the structure to obtain three relatively stable molecular conformations, as shown in Figure S18. They exhibited a high dipole moment (4.47 D, 3.40 D, and 3.06 D), indicating that, when the distance between the RM257 molecules was small, the intermolecular van der Waals force was significant. Furthermore, the calculated binding energy of two molecules with a dipole moment of 3.40 D is 0.166 eV, as shown in Figure 4B. The van der Waals force between the rod-shaped polar liquid crystal molecules increased with decreasing distance during the alignment of mesogens due to stress. The strong interaction force could offset the internal stress of the elastomer to a certain extent after the removal of the external stress. Consequently, the mesogens maintained a high-alignment state.
For this impermanent stable alignment state, the mesogens rotated slightly and did not deviate from the force range when the actuation temperature was lower than Tni. After removing the heat source, the mesogens returned to the impermanent alignment state. Moreover, when the actuation temperature was higher than Tni (64°C), the disorder of the mesogens increased until they were completely disordered, causing mesogens to fall out of the range of interaction forces. Subsequently, the mesogens remained disordered after removing the heat source. Furthermore, an LCF is stretched to 300%, and the exterior tension force is removed; it exhibited good shape memory characteristics after being placed for 20 days. After long-term placement, the permanent plasticity of the molecular network could be achieved by the relaxation of the polymer, enabling the preparation of m-LCEs.
Furthermore, we studied the effect of the intermolecular van der Waals force and the exterior force on the formation of m-LCF by observing the change in fibers with balanced components under different axial tensions. LCEs can be easily aligned by an external force as the chain extender exhibits better flexibility. Therefore, this section focuses on BDBT-LCFs. Figure 4C shows the length of the nascent BDBT-LCF as a function of time under different stresses (20, 15, 10, 4, 3, 2, and 0 kPa). The experimental procedure is illustrated in Figure S19. The fiber at 0 kPa contracted rapidly to 95.3%, and its length remained constant as the solvent continued to volatilize. Figure 4D shows the mass ratio of the residual solvent in the LCF to the completely desolvated LCF at different times after drawing BDBT-LCF fibers and the molar ratio of solvent molecules to mesogens at the same time. Figures 4C and 4D demonstrate that, under the influence of 2–20 kPa stress, the fiber length does not increase in the early solvent-volatilization stage when the degree of cross-linking of the molecular network is low. In contrast, it gradually elongated when the solvent was almost completely volatilized, and the cross-linking degree of the molecular network was high, which was unexpected. In particular, at a stress of 3 kPa, the fiber slowly elongated after 36 h with a solvent content of only 2 wt%. The solvent content implied that the origin of the alignment of mesogens in this experiment was different from that of anisotropic deswelling via controlled solvent evaporation.30 Finally, the elongation after 128 h was 64.7%. The fiber elongation was approximately 10% after 128 h at a stress of 2 kPa. A sudden change in fiber elongation was observed in the stress interval of 2–3 kPa. Furthermore, when the fiber was placed horizontally on the platform after the stress is removed, its length (initially stressed by 4–10 kPa) extended to 178%–180% of the original length after 360 h. The 2D-WAXD images of LCF stressed by 3 kPa for 128 h and removed stress for 232 h are shown in Figures S17C and S17D; the order parameters are 0.58 and 0.72, respectively.
To decrease the number of influencing factors, we selected the nascent BDBT-LCF with a diameter of 0.5 mm and a length of 35 cm. We recorded the length change at each interval (5 cm/each interval) with a load of 10 kPa during the different hang times, as shown in Figure 4F. Under the influence of the fiber weight, the upper fiber length changed rapidly. After 120 h, the load was removed, and the fiber was placed horizontally after vacuum drying. The lengths of different areas extended to 180% after 360 h, and the reversible deformation (20–100°C) was approximately 47%. The slight stress applied can guide the extension of the flexible molecular chain when the solvent is almost completely volatilized, providing space for the rotation of the liquid crystal molecules. Moreover, mesogens inevitably achieve a certain degree of stress-induced alignment along the stress direction. The van der Waals force between the mesogens dominates as the distance decreases, promoting mesogens alignment. To obtain the LCF with a high contraction ratio and simplify the continuous stress process of the nascent fiber, we matured the latter for 48 h and then performed the secondary drawing collection (80%–100%). As a result, m-LCFs with a higher directional arrangement can be obtained after a storage period. This does not require changes in external conditions or additional energy input during the process. Ten segments of fibers were randomly taken out in the BDBT-m-LCF roll to test their reversible actuation performance, as shown in Figure S20. Experimental results show that the actuation performance of the fiber is highly reproducible.
For the "two-step cross-linking method" of LCEs, a pre-stretch of 150%–200% (ΔL/L (stretch)) and a stress of 42 kPa are generally required to ensure an excellent deformation performance when mechanically stretching lightly cross-linked liquid crystal networks for the forced alignment of the mesogens (Figure S21). The synergistic effect of the van der Waals force between the molecules and the low exterior force (3 kPa) afforded the final 80% stretching (ΔL/L (stretch)) of the fiber, and its original length was restored when the actuation temperature reached Tni. Therefore, the alignment of the mesogens did not destroy the original molecular chain network. This may also be a reason for the ultrahigh strength of the fibers.
Conclusions
We proposed a new method to prepare m-LCEs with permanent plasticity utilizing the strong interaction between the stress-induced directional alignment of mesogens and the segment relaxation of LCEs. This method eliminates the adverse effects of light/heat curing and dynamic bond exchange. The DSH-m-LCFs were prepared continuously and had a reversible contraction ratio of 55.6%. Moreover, they exhibited ultrahigh breaking strength, lifting a load of 60,000 times their weight, with a maximum stress of 5.34 MPa. They overcame the limitations of existing m-LCEs in terms of size, strength, and actuation performance and exhibited a superior performance than the skeletal muscle fibers (actuation stress 0.35 MPa). The bio-muscle constructed utilizing the m-LCF and fiber bundles achieved high-load reversible deformation through thermal and light stimulation and effectively imitated the biological function of muscle contraction.
Limitations of the study
In this study, we proposed a new method and mesogens alignment mechanism to realize the continuous preparation of high-performance bionic muscle fibers based on liquid crystal elastomers. This can provide novel materials for constructing bionic muscle bundles and expand the potential applications of LCEs. However, the m-LCF developed in this study must be actuated via thermal convection, similar to the conventional m-LCE. In this regard, actuation methods, such as electrothermal control, must be further optimized. Our subsequent study will focus on strategies to ensure actuation performance and enable stretchable conduction.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| RM257 (≥97%) | Shijiazhuang Sdynano Fine Chemicals | CAS: 174063-87-7 |
| bis(3-mercaptopropionate) (≥97%) | TCI | CAS: 22504-50-3 |
| 1,4-butanediol bis(mercapto ester) (≥95%) | Aladdin | CAS: 10193-95-0 |
| bis(thioglycolate) glycol ester (≥96%) | Macklin | CAS: 123-81-9 |
| 3,6-dioxa-1,8-octanedithiol (≥97%) | Aladdin | CAS: 14970-87-7 |
| 2,2′-oxydiethanethiol (≥95%) | 9ding Chemical Technology Co., Ltd. | CAS: 2150-02-9 |
| 1,3-propanedithiol (≥98%) | Macklin | CAS: 109-80-8 |
| 1,6-hexanedithiol (≥97%) | Aladdin | CAS: 191-43-1 |
| 1,10-decanedithiol (≥96%) | Macklin | CAS: 1191-67-9 |
| pentaerythritol tetra(3-mercaptopropionate) (≥90%) | Macklin | CAS: 7575-23-7 |
| Dipropylamine(≥99%) | Macklin | CAS: 142-84-7 |
| 2,2-Dimethoxy-2-phenylacetophenone (≥99%) | Aladdin | CAS: 24650-42-8 |
| Software and algorithms | ||
| GaussView 6 | Gaussian, Inc | http://www.gaussian.com/ |
| Gaussian 09 | Gaussian, Inc | http://www.gaussian.com/ |
| Comsol Multiphysics 5.6 | Comsol Multiphysics | https://cn.comsol.com/release/5.6 |
| Origin 2018 | OriginLab | https://www.originlab.com/2018 |
| Other | ||
| Hypix-6000 direct-reading photon detector | Rigaku | https://www.rigaku.com/products/detectors/hypix6000 |
| Field-emission SEM (JSM 6700 F) | Jeol | https://speciation.net/Database/Instruments/JEOL/JSM6700F-;i25 |
| Differential scanning calorimeter (DSC8000) | Perkin Elmer | https://www.perkinelmer.com.cn/product/dsc-8000-lab-system-n5340511 |
| Uniaxial mechanical tester (QT-6203S) | QT Instrument | https://m.hbzhan.com/st546840/product_22370493.html |
| Discovery NDJ-1 rotary viscosimeter | Bang Xi Instrument Technology Co., Ltd | http://www.lichen17.com/product/show/1052.html |
| Laser displacement sensor (HG-C1200) | Panasonic | https://www3.panasonic.biz/ac/e/search_num/index.jsp?c=detail&part_no=HG-C1200 |
| High-definition camera (HD-4K) | Shenzhen Ruishishang Commerce Electronic Co., LTD | https://detail.tmall.com/item.htm?abbucket=20&id=630059701996&ns=1&spm=a230r.1.14.3.747fea2efeQKz5 |
| Temperature chamber | This paper | N/A |
| Fiber-drawing equipment | This paper | N/A |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Prof. Ningyi Yuan (nyyuan@cczu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
This study does not use experimental methods typical in the life sciences.
Method details
Synthetic method
Synthesis of m-LCFs. RM257 (3.4 mmol; 2.06 g) and a flexible chain extender (molar ratio: 1:0.95, 1:0.9, 1:0.85, and 1:0.8) were dissolved in THF (6 g). Thereafter, DPA (20 μL) was added to achieve the thiol-acrylate Michael addition reaction. After 24 h of airtight reaction at room temperature, PETMP and DPA (20 μL) were added to the prepolymer solution to realize a three-dimensional main-chain liquid crystal polymer network by cross-linking the straight-chain prepolymers. The molar ratios of RM257 and PETMP were 1:0.025, 1:0.05, 1:0.075, and 1:0.1; that is, the molar ratio of acrylate groups to thiol groups was 1:1 for the overall reaction process.
Synthesis of m-LCE films containing different amounts of excess mesogens via the two-step cross-linking method. RM257 (3.4 mmol, 2.06 g), DPA (20 μL), DMPA (0.02 g), and the flexible chain extender were dissolved in THF (6 g) and reacted. After 24 h of airtight reaction at room temperature, Pentaerythritol tetra(3-mercaptopropionate) (PETMP) and DPA (20 μL) were added to the prepolymer solution. After stirring for 30 min, the solution was placed in a Polytetrafluoroethylene (PTFE) mold and sealed. The molar ratios of RM257 to EDDET were 1:0.93, 1:0.9, 1:0.85, 1:0.8, 1:0.75, and 1:0.7, and the molar ratio of RM257 to PETMP was 1:0.05. After 24 h of airtight reaction at room temperature, they were placed in a vacuum oven at 45°C for 12 h for solvent removal. The film removed from the model was subjected to pre-stretching (0, 50%, 100%, 150%, and 200%) and irradiated using ultraviolet light (10 mW/cm2) for 10 min of light-curing.
Measurement and simulating computation
Two-dimensional-WAXD patterns were obtained using a Hypix-6000 direct-reading photon detector (HomeLab, Rigaku). The microstructure was observed using field-emission SEM (FE-SEM, Jeol JSM 6700 F). DSC curves were obtained using a differential scanning calorimeter (DSC8000, Perkin Elmer) at a heating rate of 20°C min−1 and a cooling rate of 5°C min−1. Tensile tests were conducted using a uniaxial mechanical tester (QT-6203S, QT Instrument) with a load cell of 2000 N. The rheological performance was evaluated using a Discovery NDJ-1 rotary viscosimeter (Bang Xi Instrument Technology Co., Ltd, Shanghai). The actuation deformation was tested using a laser displacement sensor (HG-C1200, Panasonic; positioning accuracy: 200 μm). The images and videos were recorded using a high-definition camera (HD-4K). The temperature chamber was custom-made, and its temperature control accuracy was 0.1°C. The temperature around the sample in the temperature chamber was measured using a thermocouple with an accuracy of 0.01°C.
We used density functional theory to conduct a theoretical calculation analysis of the liquid crystal molecule (RM257). The structure was established, and theoretical calculations were performed using the GaussView 6 and Gaussian 09 package. The geometries of RM257 were optimized using the exchange-correlation functional (B3LYP) with the 6-311G basis sets. The dipole moments were also calculated.
The binding energy (Eb) of two RM257 molecules was calculated as follows:
| Eb = 2 ∗ ERM257 – ERM257-2 – E_BSSE |
where E RM257 is the total energy of a single RM257 molecule; ERM257-2 is the total energy of the system of the two RM257 molecules, and E_BSSE is the Basis Set Superposition Error (BSSE).
We combined the finite element method with the level-set method to investigate the deformation during the fiber-drawing process. The Comsol Multiphysics 5.6 software was used to simulate this process. We assumed a laminar flow model owing to the low drawing velocity and small Reynolds number. The flow, pressure, and velocity fields were calculated based on this model. We built a level-set to study and trace the deformation of the LCE during the entire process.
Quantification and statistical analysis
Quantification and statistical analysis were performed by Origin 2018.
Acknowledgments
This work was supported by the Special fund for Science and technology innovation of Jiangsu Province (BE2022610), Jiangsu Provincial "333" High-level Talent Training Project.
Author contributions
Xu Dong: Conceptualization, investigation, writing - original draft. Xiaoshuang Zhou: Investigation, writing - original draft. Lvzhou Li: Validation, writing - review & editing. Xiaoting Cao: Methodology, writing - original draft. Jiawei Xu: Methodology, writing - review & editing. Shengping Dai: Investigation. Yaoyao Jiang: Investigation. Qingyue Li: Investigation. Ningyi Yuan: Conceptualization, resources, supervision, writing - review & editing. Jianning Ding: Funding acquisition, resources, supervision, writing - review & editing.
Declaration of interests
The authors declare no competing interests.
Published: March 9, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106357.
Contributor Information
Lvzhou Li, Email: eastoasis7@163.com.
Ningyi Yuan, Email: nyyuan@cczu.edu.cn.
Jianning Ding, Email: dingjn@yzu.edu.cn.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request
References
- 1.Huang Y., Zheng N., Cheng Z., Chen Y., Lu B., Xie T., Feng X. Direct laser writing-based programmable transfer printing via bioinspired shape memory reversible adhesive. ACS Appl. Mater. Interfaces. 2016;8:35628–35633. doi: 10.1021/acsami.6b11696. [DOI] [PubMed] [Google Scholar]
- 2.Lendlein A., Behl M., Hiebl B., Wischke C. Shape-memory polymers as A technology platform for biomedical applications. Expert Rev. Med. Devices. 2010;7:357–379. doi: 10.1586/erd.10.8. [DOI] [PubMed] [Google Scholar]
- 3.Kanik M., Orguc S., Varnavides G., Kim J., Benavides T., Gonzalez D., Akintilo T., Tasan C.C., Chandrakasan A.P., Fink Y., Anikeeva P. Strain-programmable fiber-based artificial muscle. Science. 2019;365:145–150. doi: 10.1126/science.aaw2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunget G., Seelecke S. Actuator placement for A bio-inspired bone-joint system based on SMA. Active and Passive Smart Structures and Integrated Systems 2009. SPIE, 2009;7288:259–270. doi: 10.1117/12.816309. [DOI] [Google Scholar]
- 5.Tu Z., Liu W., Wang J., Qiu X., Huang J., Li J., Lou H. Biomimetic high performance artificial muscle built on sacrificial coordination network and mechanical training process. Nat. Commun. 2021;12:2916–2927. doi: 10.1038/s41467-021-23204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behl M., Kratz K., Zotzmann J., Nöchel U., Lendlein A. Reversible bidirectional shape-memory polymers. Adv. Mater. 2013;25:4466–4469. doi: 10.1002/adma.201300880. [DOI] [PubMed] [Google Scholar]
- 7.Song H., Fang Z., Jin B., Pan P., Zhao Q., Xie T. Synergetic chemical and physical programming for reversible shape memory effect in A dynamic covalent network with two crystalline phases. ACS Macro Lett. 2019;8:682–686. doi: 10.1021/acsmacrolett.9b00291. [DOI] [PubMed] [Google Scholar]
- 8.Zeng H., Wani O.M., Wasylczyk P., Priimagi A. Light-driven, caterpillar-inspired miniature inching robot. Macromol. Rapid Commun. 2018;39:1700224. doi: 10.1002/marc.201700224. [DOI] [PubMed] [Google Scholar]
- 9.Komp A., Rühe J., Finkelmann H. A versatile preparation route for thin fre-tanding liquid single crystal elastomers. Macromol. Rapid Commun. 2005;26:813–818. doi: 10.1002/marc.200500049. [DOI] [Google Scholar]
- 10.He Q., Wang Z., Wang Y., Minori A., Tolley M.T., Cai S. Electrically controlled liquid crystal elastomer–based soft tubular actuator with multimodal actuation. Sci. Adv. 2019;5:eaax5746. doi: 10.1126/sciadv.aax5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yakacki C.M., Saed M., Nair D.P., Gong T., Reed S.M., Bowman C.N. Tailorable and programmable liquid-crystalline elastomers using a two-stage thiol–acrylate reaction. RSC Adv. 2015;5:18997–19001. doi: 10.1039/C5RA01039J. [DOI] [Google Scholar]
- 12.McBride M.K., Martinez A.M., Cox L., Alim M., Childress K., Beiswinger M., Podgorski M., Worrell B.T., Killgore J., Bowman C.N. A readily programmable, fully reversible shape-switching material. Sci. Adv. 2018;4:eaat4634. doi: 10.1126/sciadv.aat4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson E.C., Kotikian A., Li S., Aizenberg J., Lewis J.A. 3D printable and reconfigurable liquid crystal elastomers with light-induced shape memory via dynamic bond exchange. Adv. Mater. 2020;32:1905682. doi: 10.1002/adma.201905682. [DOI] [PubMed] [Google Scholar]
- 14.He Q., Wang Z., Wang Y., Wang Z., Li C., Annapooranan R., Zeng J., Chen R., Cai S. Electrospun liquid crystal elastomer microfiber actuator. Sci. Robot. 2021;6:eabi9704. doi: 10.1126/scirobotics.abi9704. [DOI] [PubMed] [Google Scholar]
- 15.Kotikian A., Morales J.M., Lu A., Mueller J., Davidson Z.S., Boley J.W., Lewis J.A. Innervated, self-sensing liquid crystal elastomer actuators with closed loop control. Adv. Mater. 2021;33:2101814. doi: 10.1002/adma.202101814. [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Gao Y., Wang H., Poling-Skutvik R., Osuji C.O., Yang S. Shaping and locomotion of soft robots using filament actuators made from liquid crystal elastomer–carbon nanotube composites. Adv. Intell. Syst. 2020;2:1900163. doi: 10.1002/aisy.201900163. [DOI] [Google Scholar]
- 17.Li S., Bai H., Liu Z., Zhang X., Huang C., Wiesner L.W., Silberstein M., Shepherd R.F. Digital light processing of liquid crystal elastomers for self-sensing artificial muscles. Sci. Adv. 2021;7:eabg3677. doi: 10.1126/sciadv.abg3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomsen D.L., Keller P., Naciri J., Pink R., Jeon H., Shenoy D., Ratna B.R. Liquid crystal elastomers with mechanical properties of A muscle. Macromolecules. 2001;34:5868–5875. doi: 10.1021/ma001639q. [DOI] [Google Scholar]
- 19.Liu L., Liu M.-H., Deng L.-L., Lin B.-P., Yang H. Near-infrared chromophore functionalized soft actuator with ultrafast photoresponsive speed and superior mechanical property. J. Am. Chem. Soc. 2017;139:11333–11336. doi: 10.1021/jacs.7b06410. [DOI] [PubMed] [Google Scholar]
- 20.Yang R., Zhao Y. Multitemperature memory actuation of A liquid crystal polymer network over A broad nematic–isotropic phase transition induced by large strain. ACS Macro Lett. 2018;7:353–357. doi: 10.1021/acsmacrolett.8b00089. [DOI] [PubMed] [Google Scholar]
- 21.Ishige R., Osada K., Tagawa H., Niwano H., Tokita M., Watanabe J. Elongation behavior of A main-chain smectic liquid crystalline elastomer. Macromolecules. 2008;41:7566–7570. doi: 10.1021/ma801665a. [DOI] [Google Scholar]
- 22.Urayama K., Mashita R., Kobayashi I., Takigawa T. Stretching-induced director rotation in thin films of liquid crystal elastomers with homeotropic alignment. Macromolecules. 2007;40:7665–7670. doi: 10.1021/ma071104y. [DOI] [Google Scholar]
- 23.Naciri J., Srinivasan A., Jeon H., Nikolov N., Keller P., Ratna B.R. Nematic elastomer fiber actuator. Macromolecules. 2003;36:8499–8505. doi: 10.1021/ma034921g. [DOI] [Google Scholar]
- 24.Michal B.T., McKenzie B.M., Felder S.E., Rowan S.J. Metallo-Thermo-and photoresponsive shape memory and actuating liquid crystalline elastomers. Macromolecules. 2015;48:3239–3246. doi: 10.1021/acs.macromol.5b00646. [DOI] [Google Scholar]
- 25.Wang L., Liu W., Guo L.-X., Lin B.-P., Zhang X.-Q., Sun Y., Yang H. A room-temperature two-stage thiol–ene photoaddition approach towards monodomain liquid crystalline elastomers. Polym. Chem. 2017;8:1364–1370. doi: 10.1039/C6PY02096H. [DOI] [Google Scholar]
- 26.Liu L., Wang M., Guo L.-X., Sun Y., Zhang X.-Q., Lin B.-P., Yang H. Aggregation-induced emission luminogen-functionalized liquid crystal elastomer soft actuators. Macromolecules. 2018;51:4516–4524. doi: 10.1021/acs.macromol.8b00677. [DOI] [Google Scholar]
- 27.Lu H.-F., Wang M., Chen X.-M., Lin B.-P., Yang H. Interpenetrating liquid-crystal polyurethane/polyacrylate elastomer with ultrastrong mechanical property. J. Am. Chem. Soc. 2019;141:14364–14369. doi: 10.1021/jacs.9b06757. [DOI] [PubMed] [Google Scholar]
- 28.Saed M.O., Ambulo C.P., Kim H., De R., Raval V., Searles K., Siddiqui D.A., Cue J.M.O., Stefan M.C., Shankar M.R., Ware T.H. Molecularly-engineered, 4D-printed liquid crystal elastomer actuators. Adv. Funct. Mater. 2019;29:1806412. doi: 10.1002/adfm.201806412. [DOI] [Google Scholar]
- 29.Ren L., Li B., He Y., Song Z., Zhou X., Liu Q., Ren L. Programming shape-morphing behavior of liquid crystal elastomers via parameter-encoded 4D printing. ACS Appl. Mater. Interfaces. 2020;12:15562–15572. doi: 10.1021/acsami.0c00027. [DOI] [PubMed] [Google Scholar]
- 30.Jin B., Liu J., Shi Y., Chen G., Zhao Q., Yang S. Solvent-assisted 4D programming and reprogramming of liquid crystalline organogels. Adv. Mater. 2022;34:2107855. doi: 10.1002/adma.20210785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request